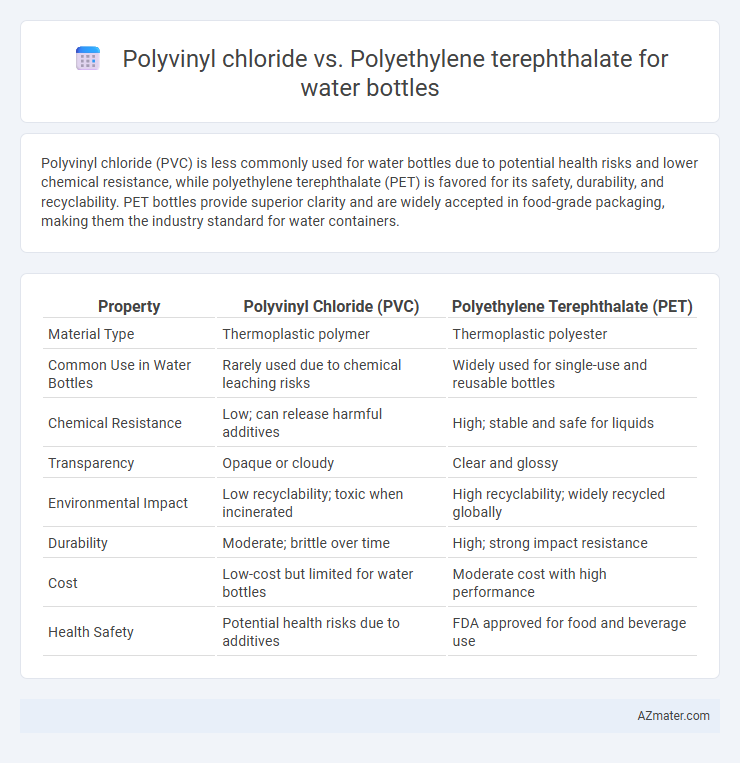
Polyvinyl chloride (PVC) is less commonly used for water bottles due to potential health risks and lower chemical resistance, while polyethylene terephthalate (PET) is favored for its safety, durability, and recyclability. PET bottles provide superior clarity and are widely accepted in food-grade packaging, making them the industry standard for water containers.
Table of Comparison
| Property | Polyvinyl Chloride (PVC) | Polyethylene Terephthalate (PET) |
|---|---|---|
| Material Type | Thermoplastic polymer | Thermoplastic polyester |
| Common Use in Water Bottles | Rarely used due to chemical leaching risks | Widely used for single-use and reusable bottles |
| Chemical Resistance | Low; can release harmful additives | High; stable and safe for liquids |
| Transparency | Opaque or cloudy | Clear and glossy |
| Environmental Impact | Low recyclability; toxic when incinerated | High recyclability; widely recycled globally |
| Durability | Moderate; brittle over time | High; strong impact resistance |
| Cost | Low-cost but limited for water bottles | Moderate cost with high performance |
| Health Safety | Potential health risks due to additives | FDA approved for food and beverage use |
Introduction to Polyvinyl Chloride and Polyethylene Terephthalate
Polyvinyl chloride (PVC) is a durable synthetic polymer widely used in packaging due to its chemical resistance and cost-effectiveness, though it has limited flexibility and environmental concerns. Polyethylene terephthalate (PET), a thermoplastic polymer resin, is favored for water bottles because of its lightweight nature, transparency, and recyclability. PET's superior barrier properties and safer consumption profile often make it the preferred material over PVC in beverage packaging.
Chemical Composition and Structure Comparison
Polyvinyl chloride (PVC) consists of repeating vinyl chloride units with a chlorine atom attached to every other carbon, providing rigidity and chemical resistance due to its polar C-Cl bonds. Polyethylene terephthalate (PET) is a polyester formed by the condensation polymerization of terephthalic acid and ethylene glycol, featuring ester linkages and aromatic rings that create a semi-crystalline structure offering high strength and clarity. While PVC's PVC backbone yields flexibility with moderate barrier properties, PET's molecular arrangement results in excellent gas and moisture barrier resistance, making it more suitable for water bottle applications.
Manufacturing Processes Overview
Polyvinyl chloride (PVC) and Polyethylene terephthalate (PET) differ significantly in their manufacturing processes for water bottles. PVC production involves polymerizing vinyl chloride monomers through suspension or emulsion polymerization, followed by extrusion or blow molding to shape the bottles, which often requires plasticizers to enhance flexibility. PET manufacturing utilizes polycondensation of terephthalic acid and ethylene glycol, creating a lightweight, strong polymer that undergoes injection molding and stretch blow molding, resulting in highly transparent and durable water bottles.
Physical Properties: Strength and Durability
Polyvinyl chloride (PVC) offers excellent strength and rigidity, making it highly resistant to impact and environmental stress, which contributes to its durability as a water bottle material. Polyethylene terephthalate (PET) is lightweight with good tensile strength and superior resistance to cracking and punctures, favored for its flexibility and transparency in water bottles. PET generally outperforms PVC in terms of durability under repeated use and exposure to heat, maintaining integrity without significant degradation.
Weight and Design Flexibility
Polyvinyl chloride (PVC) is heavier than polyethylene terephthalate (PET), making PET a preferred choice for lightweight water bottles that reduce transportation costs. PET offers superior design flexibility with better transparency and ease of molding, allowing for a wide variety of bottle shapes and sizes. While PVC provides durability and chemical resistance, PET's weight efficiency and customizable design options make it more suitable for mass-market water bottle production.
Safety and Health Implications
Polyvinyl chloride (PVC) water bottles often raise safety concerns due to the potential leaching of harmful chemicals like phthalates and chlorine compounds, which can disrupt endocrine functions and pose health risks. In contrast, polyethylene terephthalate (PET) is widely regarded as safer for water storage because it is less likely to leach harmful substances under normal usage conditions and is approved by regulatory agencies like the FDA for food and beverage contact. Choosing PET over PVC for water bottles significantly reduces exposure to toxic additives and better supports consumer health and safety standards.
Environmental Impact and Recyclability
Polyvinyl chloride (PVC) water bottles pose significant environmental challenges due to their toxic chemical additives and difficulty in recycling, often leading to harmful landfill accumulation and hazardous emissions. Polyethylene terephthalate (PET) bottles, widely used for water packaging, offer superior recyclability through established curbside programs and recycling facilities, significantly reducing landfill waste and supporting circular economy initiatives. PET's lower environmental footprint and higher recycling rate make it a more sustainable choice compared to PVC for water bottle production.
Cost Factors and Market Availability
Polyvinyl chloride (PVC) generally offers lower production costs compared to polyethylene terephthalate (PET), making it a more cost-effective option for water bottle manufacturing. However, PET dominates the market due to its superior recyclability, chemical resistance, and consumer preference for safer, BPA-free materials. Market availability of PET bottles is significantly higher worldwide, supported by established recycling infrastructures and widespread industry adoption, whereas PVC bottles face restrictions and lower demand due to environmental and health concerns.
Performance in Water Storage and Shelf Life
Polyvinyl chloride (PVC) offers excellent chemical resistance and a strong barrier against gas permeability, making it effective for water storage but has potential leaching concerns. Polyethylene terephthalate (PET) provides superior clarity, higher impact resistance, and is lightweight with excellent moisture and gas barrier properties, resulting in a longer shelf life for bottled water. PET is widely preferred in the beverage industry due to its safer profile, recyclability, and ability to maintain water quality over extended storage periods.
Choosing the Right Material for Water Bottles
Polyethylene terephthalate (PET) is the preferred material for water bottles due to its lightweight nature, excellent clarity, and strong resistance to impact, making it ideal for single-use and recyclable bottles. Polyvinyl chloride (PVC), while durable and chemically resistant, is less commonly used for water bottles because it can release harmful chemicals and is harder to recycle. Choosing PET over PVC ensures safer, more environmentally friendly water bottles that meet food safety standards and consumer health concerns.
Infographic: Polyvinyl chloride vs Polyethylene terephthalate for Water Bottle
 azmater.com
azmater.com