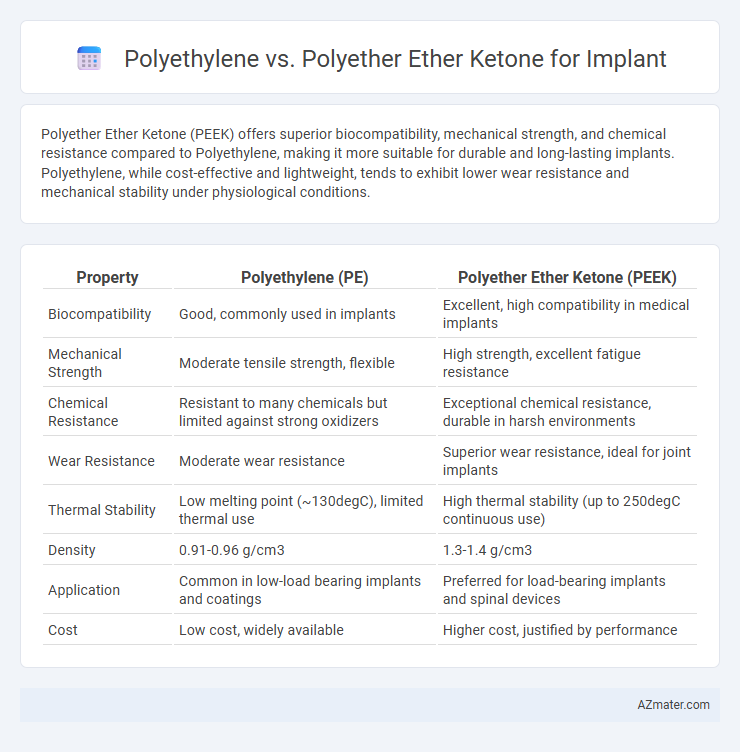
Polyether Ether Ketone (PEEK) offers superior biocompatibility, mechanical strength, and chemical resistance compared to Polyethylene, making it more suitable for durable and long-lasting implants. Polyethylene, while cost-effective and lightweight, tends to exhibit lower wear resistance and mechanical stability under physiological conditions.
Table of Comparison
| Property | Polyethylene (PE) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Biocompatibility | Good, commonly used in implants | Excellent, high compatibility in medical implants |
| Mechanical Strength | Moderate tensile strength, flexible | High strength, excellent fatigue resistance |
| Chemical Resistance | Resistant to many chemicals but limited against strong oxidizers | Exceptional chemical resistance, durable in harsh environments |
| Wear Resistance | Moderate wear resistance | Superior wear resistance, ideal for joint implants |
| Thermal Stability | Low melting point (~130degC), limited thermal use | High thermal stability (up to 250degC continuous use) |
| Density | 0.91-0.96 g/cm3 | 1.3-1.4 g/cm3 |
| Application | Common in low-load bearing implants and coatings | Preferred for load-bearing implants and spinal devices |
| Cost | Low cost, widely available | Higher cost, justified by performance |
Introduction to Polyethylene and Polyether Ether Ketone
Polyethylene is a versatile thermoplastic polymer widely used in medical implants due to its excellent biocompatibility, chemical resistance, and flexibility. Polyether ether ketone (PEEK) is a high-performance engineering thermoplastic known for its remarkable mechanical strength, thermal stability, and resistance to wear, making it ideal for demanding biomedical applications. Both materials offer distinct advantages in implant design, with polyethylene favored for cushioning and flexibility, while PEEK is preferred for load-bearing structural components.
Material Composition and Structure
Polyethylene, primarily composed of long chains of ethylene monomers, exhibits a semi-crystalline structure that provides flexibility and resistance to wear, making it a popular choice for orthopedic implants like joint replacements. Polyether Ether Ketone (PEEK) features a highly crystalline aromatic polymer structure with repeating ether and ketone groups, granting superior mechanical strength, chemical resistance, and biocompatibility ideal for spinal cages and dental implants. The distinct molecular architecture of PEEK results in enhanced stiffness and thermal stability compared to the more ductile and lower modulus polyethylene.
Mechanical Properties Comparison
Polyether Ether Ketone (PEEK) exhibits superior mechanical properties compared to Polyethylene, including higher tensile strength, enhanced stiffness, and excellent resistance to wear and fatigue, making it ideal for load-bearing implants. Polyethylene, while having good impact resistance and flexibility, tends to have lower modulus and decreased durability under prolonged mechanical stress. PEEK's biocompatibility and dimensional stability under physiological conditions provide significant advantages in orthopedic and dental implant applications.
Biocompatibility and Bioactivity
Polyether ether ketone (PEEK) demonstrates superior biocompatibility compared to polyethylene, showing minimal inflammatory response and excellent tissue integration, which is critical for long-term implant success. While polyethylene offers adequate biocompatibility, its bioactivity is limited, often requiring surface modifications to enhance osseointegration. PEEK's inherent bioactivity, combined with its mechanical properties closely resembling cortical bone, makes it a preferred choice for implants demanding both stability and biological interaction.
Wear Resistance and Longevity
Polyether ether ketone (PEEK) exhibits superior wear resistance compared to polyethylene, making it more suitable for long-term implant applications where durability is critical. PEEK's high mechanical strength and chemical stability contribute to enhanced longevity and reduced maintenance in orthopedic and dental implants. Polyethylene, while biocompatible, tends to degrade faster under repeated mechanical stress, limiting its lifespan in high-load environments.
Clinical Performance in Implants
Polyether ether ketone (PEEK) demonstrates superior clinical performance compared to polyethylene in implants due to its enhanced mechanical strength, biocompatibility, and resistance to wear and chemical degradation. Clinical studies reveal that PEEK implants exhibit lower rates of inflammation and improved osseointegration, making them preferable for load-bearing orthopedic and dental applications. Polyethylene implants, while cost-effective, often face challenges such as wear particle-induced osteolysis and reduced longevity in high-stress environments.
Imaging Compatibility (MRI & X-ray)
Polyethylene exhibits excellent imaging compatibility with minimal artifacts in both MRI and X-ray, making it ideal for precise postoperative assessments of implants. Polyether Ether Ketone (PEEK), while biocompatible and mechanically robust, shows moderate MRI artifacts due to its semi-crystalline structure but remains radiolucent on X-rays, facilitating clearer bone visualization. Selecting polyethylene or PEEK depends on the specific imaging requirements for implant monitoring and diagnostic clarity.
Cost and Manufacturing Considerations
Polyethylene offers a lower-cost option for implants, benefiting from widespread availability and simpler manufacturing processes such as molding and machining. In contrast, Polyether Ether Ketone (PEEK) commands higher costs due to its advanced chemical structure and requires specialized manufacturing techniques like high-temperature extrusion and precision CNC machining. While PEEK provides superior mechanical strength and biocompatibility, polyethylene remains favored for cost-sensitive applications with less demanding performance requirements.
Regulatory Approvals and Standards
Polyethylene (PE) and Polyether Ether Ketone (PEEK) used in implants differ significantly in regulatory approvals and standards. PE, particularly Ultra-High Molecular Weight Polyethylene (UHMWPE), has long-standing FDA clearance for orthopedic implants due to its proven biocompatibility and wear resistance, conforming to ISO 5834 and ASTM F648 standards. Conversely, PEEK meets stringent FDA and ISO 10993 biocompatibility requirements for spinal and cranial implants, offering superior mechanical properties and thermal stability, yet often requires more rigorous testing to achieve regulatory clearance for specific implant applications.
Future Trends in Implant Materials
Polyether Ether Ketone (PEEK) is gaining significant traction over traditional Polyethylene in implant materials due to its superior mechanical strength, biocompatibility, and resistance to wear and chemical degradation. Future trends indicate a rise in the development of composite PEEK implants infused with bioactive nanoparticles to enhance osseointegration and antimicrobial properties. Advances in additive manufacturing and surface modification techniques are expected to further customize PEEK implants, optimizing long-term performance and patient-specific applications.
Infographic: Polyethylene vs Polyether Ether Ketone for Implant
 azmater.com
azmater.com