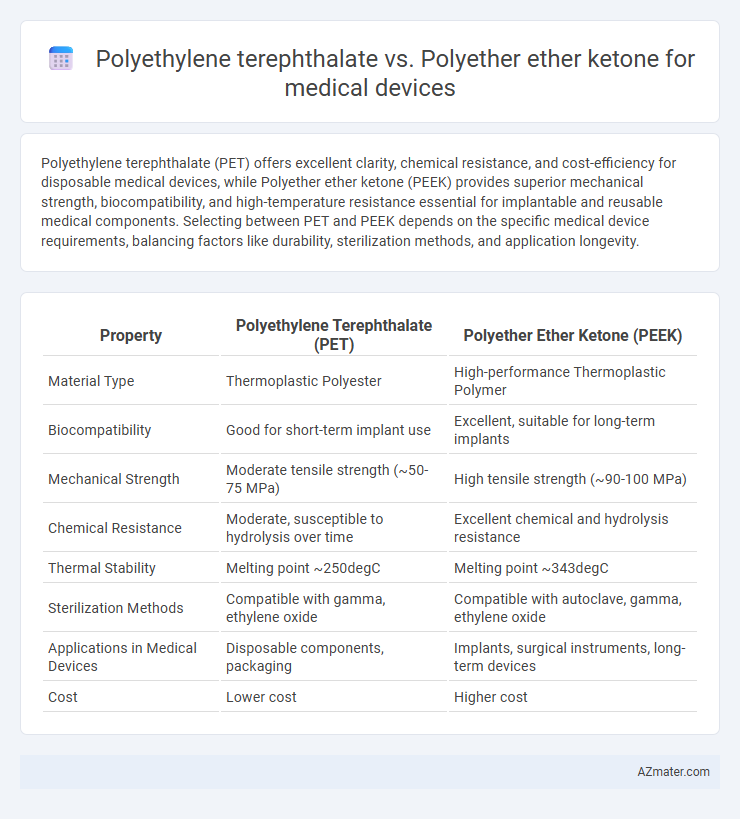
Polyethylene terephthalate (PET) offers excellent clarity, chemical resistance, and cost-efficiency for disposable medical devices, while Polyether ether ketone (PEEK) provides superior mechanical strength, biocompatibility, and high-temperature resistance essential for implantable and reusable medical components. Selecting between PET and PEEK depends on the specific medical device requirements, balancing factors like durability, sterilization methods, and application longevity.
Table of Comparison
| Property | Polyethylene Terephthalate (PET) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Thermoplastic Polyester | High-performance Thermoplastic Polymer |
| Biocompatibility | Good for short-term implant use | Excellent, suitable for long-term implants |
| Mechanical Strength | Moderate tensile strength (~50-75 MPa) | High tensile strength (~90-100 MPa) |
| Chemical Resistance | Moderate, susceptible to hydrolysis over time | Excellent chemical and hydrolysis resistance |
| Thermal Stability | Melting point ~250degC | Melting point ~343degC |
| Sterilization Methods | Compatible with gamma, ethylene oxide | Compatible with autoclave, gamma, ethylene oxide |
| Applications in Medical Devices | Disposable components, packaging | Implants, surgical instruments, long-term devices |
| Cost | Lower cost | Higher cost |
Introduction: Polyethylene Terephthalate vs Polyether Ether Ketone in Medical Devices
Polyethylene terephthalate (PET) and polyether ether ketone (PEEK) are prominent polymers used in medical devices due to their distinct material properties. PET offers excellent chemical resistance and transparency, making it suitable for disposable medical packaging and diagnostic components, while PEEK provides superior mechanical strength, biocompatibility, and high-temperature resistance, ideal for implantable devices and surgical instruments. The choice between PET and PEEK hinges on specific application requirements, balancing factors such as durability, sterilization methods, and long-term performance in biological environments.
Material Overview: Properties of PET and PEEK
Polyethylene terephthalate (PET) offers excellent chemical resistance, high tensile strength, and dimensional stability, making it suitable for disposable medical devices and packaging applications. Polyether ether ketone (PEEK) exhibits superior mechanical properties, outstanding thermal resistance up to 250degC, and biocompatibility, ideal for implantable devices and sterilizable medical components. PET is favored for cost-effective, single-use applications, while PEEK provides exceptional durability and longevity in demanding medical environments.
Biocompatibility Comparison: PET vs PEEK
Polyethylene terephthalate (PET) and Polyether ether ketone (PEEK) are widely used polymers in medical devices, with notable differences in biocompatibility. PET exhibits excellent biostability and is commonly used in applications requiring long-term implant stability, but it may induce mild inflammatory responses in some tissues. PEEK offers superior biocompatibility with minimal cytotoxicity and excellent resistance to bodily fluids, making it ideal for load-bearing implants requiring high mechanical strength and prolonged biocompatibility.
Mechanical Strength and Durability Analysis
Polyether ether ketone (PEEK) exhibits superior mechanical strength and durability compared to polyethylene terephthalate (PET), making it highly suitable for demanding medical device applications requiring high tensile strength and resistance to wear. PEEK offers exceptional fatigue resistance and maintains structural integrity under repeated sterilization cycles, while PET tends to have lower tensile strength and can degrade with prolonged exposure to harsh sterilization processes. The enhanced chemical resistance and thermal stability of PEEK contribute to its longer lifespan and reliability in critical medical environments compared to PET.
Sterilization Compatibility and Chemical Resistance
Polyether ether ketone (PEEK) offers superior sterilization compatibility for medical devices, withstanding repeated autoclaving, gamma radiation, and ethylene oxide without degradation, whereas polyethylene terephthalate (PET) has limited resistance to high-temperature sterilization methods. PEEK demonstrates exceptional chemical resistance to acids, bases, and organic solvents, making it ideal for harsh medical environments, while PET is more susceptible to hydrolysis and chemical attack. These properties position PEEK as the preferred material for sterilization-intensive and chemically demanding medical device applications.
Radiolucency and Imaging Performance
Polyethylene terephthalate (PET) exhibits moderate radiolucency suitable for certain medical device applications requiring clear imaging without significant artifact interference. Polyether ether ketone (PEEK) offers superior radiolucency, enabling enhanced imaging performance in modalities such as X-ray, CT, and MRI, crucial for precision in medical diagnostics and interventions. The choice of PEEK over PET significantly improves visualization of implants and surrounding tissues, reducing the risk of imaging obstructions and improving post-operative assessment accuracy.
Cost Implications for Medical Manufacturers
Polyethylene terephthalate (PET) offers a cost-effective solution for medical manufacturers due to its lower raw material price and ease of processing compared to polyether ether ketone (PEEK). PEEK's high-performance properties come with a significantly higher price point and increased manufacturing complexity, impacting overall production budgets. Choosing PET can reduce manufacturing expenses while maintaining acceptable performance for non-critical medical device components.
Applications: Clinical Uses of PET and PEEK
Polyethylene terephthalate (PET) is widely used in medical devices for applications such as flexible tubes, sutures, and vascular grafts due to its excellent biocompatibility and mechanical strength. Polyether ether ketone (PEEK) is preferred in orthopedic implants, spinal fusion devices, and dental applications for its superior chemical resistance, high-temperature stability, and radiolucency. Clinical use of PET centers on soft tissue engineering and fluid delivery systems, while PEEK is favored for load-bearing implants and long-term structural support in demanding medical environments.
Regulatory Approvals and Standards Compliance
Polyethylene terephthalate (PET) and Polyether ether ketone (PEEK) are widely used polymers in medical device manufacturing, each with distinct regulatory approvals and standards compliance profiles. PET is approved by the FDA for various medical applications due to its biocompatibility and clarity, complying with ISO 10993 for biological evaluation of medical devices, making it suitable for disposable syringes and packaging. PEEK meets stringent FDA and USP Class VI certifications, excels in sterilization processes, and adheres to ISO 13485 for medical device quality management, positioning it as a preferred material in implantable devices and high-performance surgical instruments.
Future Perspectives: Innovations in Medical Device Materials
Polyether ether ketone (PEEK) offers superior chemical resistance, biocompatibility, and mechanical strength compared to polyethylene terephthalate (PET), making it increasingly preferred for advanced medical devices such as implants and surgical instruments. Innovations in additive manufacturing and surface modification techniques are driving the adoption of PEEK in applications requiring long-term durability and sterilization resilience. Future developments are likely to focus on enhancing PEEK's bioactivity and integrating antimicrobial properties to meet evolving regulatory standards and clinical demands.
Infographic: Polyethylene terephthalate vs Polyether ether ketone for Medical Device
 azmater.com
azmater.com