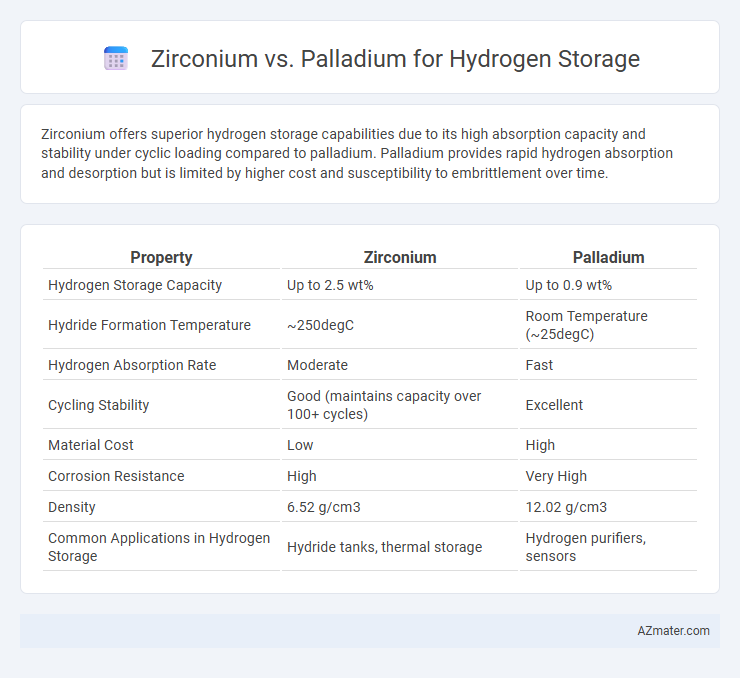
Zirconium offers superior hydrogen storage capabilities due to its high absorption capacity and stability under cyclic loading compared to palladium. Palladium provides rapid hydrogen absorption and desorption but is limited by higher cost and susceptibility to embrittlement over time.
Table of Comparison
| Property | Zirconium | Palladium |
|---|---|---|
| Hydrogen Storage Capacity | Up to 2.5 wt% | Up to 0.9 wt% |
| Hydride Formation Temperature | ~250degC | Room Temperature (~25degC) |
| Hydrogen Absorption Rate | Moderate | Fast |
| Cycling Stability | Good (maintains capacity over 100+ cycles) | Excellent |
| Material Cost | Low | High |
| Corrosion Resistance | High | Very High |
| Density | 6.52 g/cm3 | 12.02 g/cm3 |
| Common Applications in Hydrogen Storage | Hydride tanks, thermal storage | Hydrogen purifiers, sensors |
Introduction to Hydrogen Storage Materials
Zirconium and Palladium are critical materials in the realm of hydrogen storage due to their unique atomic structures and absorption capacities. Zirconium alloys offer high hydrogen storage density with excellent corrosion resistance, making them suitable for solid-state hydrogen storage systems. Palladium exhibits exceptional hydrogen solubility and reversible absorption properties, often used in hydrogen purification and sensor applications.
Overview of Zirconium and Its Properties
Zirconium exhibits high corrosion resistance and exceptional mechanical strength, making it a prime candidate for hydrogen storage applications. Its ability to form stable metal hydrides enables efficient hydrogen absorption and release, essential for energy storage technologies. The metal's low neutron absorption cross-section also allows its use in nuclear reactors, highlighting its versatility beyond hydrogen storage.
Overview of Palladium and Its Properties
Palladium is a transition metal renowned for its exceptional ability to absorb hydrogen, storing up to 900 times its volume in a solid-state form. Its face-centered cubic (FCC) crystal structure facilitates hydrogen diffusion and reversible absorption, making it a prime candidate for hydrogen storage applications. The metal's high catalytic activity and resistance to corrosion further enhance its suitability for efficient hydrogen uptake and release.
Hydrogen Absorption Mechanisms in Zirconium
Zirconium absorbs hydrogen through a dissociative chemisorption mechanism where hydrogen molecules split into atoms and diffuse into the metal lattice, forming zirconium hydrides that enable high hydrogen storage capacity. The hexagonal close-packed (hcp) structure of zirconium allows efficient hydrogen diffusion and storage by accommodating interstitial hydrogen atoms without significant lattice distortion. In contrast, palladium mainly uses surface absorption with rapid hydrogen dissociation and absorption but forms more brittle hydrides, affecting its long-term hydrogen storage stability.
Hydrogen Absorption Mechanisms in Palladium
Palladium exhibits exceptional hydrogen absorption due to its unique ability to dissociate molecular hydrogen into atomic hydrogen, which then diffuses into the metal lattice, forming palladium hydride. This process involves the occupation of interstitial sites within the face-centered cubic structure, enabling high hydrogen solubility and fast kinetics. In contrast, zirconium stores hydrogen primarily through the formation of stable metal hydrides with less reversible absorption and slower diffusion rates, making palladium more efficient for dynamic hydrogen storage applications.
Storage Capacity Comparison: Zirconium vs Palladium
Zirconium alloys exhibit higher hydrogen storage capacity compared to palladium, with zirconium capable of absorbing hydrogen up to 2.0 wt%, while palladium typically stores around 0.9 wt%. The larger atomic radius and crystal structure of zirconium facilitate greater hydrogen solubility and diffusion rates than palladium's face-centered cubic lattice. Zirconium's superior storage capacity makes it more suitable for large-scale hydrogen storage applications, despite palladium's advantages in catalytic activity.
Cost and Availability Considerations
Zirconium offers a cost-effective alternative to palladium for hydrogen storage due to its abundant availability and lower market price, making it suitable for large-scale applications. Palladium, while more expensive and rarer, exhibits superior hydrogen absorption capacity and faster kinetics but is limited by its high cost and scarcity. Evaluating cost-performance ratios favors zirconium for budget-sensitive projects, whereas palladium remains preferred when efficiency and storage capacity are prioritized despite the premium price.
Durability and Cycling Performance
Zirconium alloys exhibit superior durability and stable cycling performance for hydrogen storage due to their high resistance to embrittlement and corrosion under repeated hydrogen absorption and desorption cycles. Palladium, while highly efficient in hydrogen absorption, suffers from quicker degradation and reduced cycling stability because of its susceptibility to surface poisoning and lattice distortion over time. Zirconium's robust microstructure enables maintaining consistent storage capacity and mechanical integrity through extensive hydrogen charge-discharge cycles, making it preferable for long-term hydrogen storage applications.
Safety and Environmental Impact
Zirconium-based materials offer superior safety in hydrogen storage due to their low flammability and resistance to embrittlement compared to palladium. Palladium, while highly efficient in hydrogen absorption, poses environmental concerns related to its toxic mining processes and limited recyclability. Zirconium's abundance and lower toxicity contribute to a reduced environmental footprint, making it a safer and more sustainable choice for hydrogen storage applications.
Future Prospects and Research Directions
Zirconium exhibits promising hydrogen storage capabilities due to its high absorption capacity and excellent resistance to corrosion, making it a key focus for future material innovation. Palladium, renowned for its exceptional hydrogen absorption kinetics and selective permeability, continues to drive research in hydrogen purification and catalytic applications. Emerging studies prioritize alloying these metals and nanostructuring techniques to enhance storage efficiency, stability, and cost-effectiveness for scalable hydrogen energy systems.
Infographic: Zirconium vs Palladium for Hydrogen Storage
 azmater.com
azmater.com