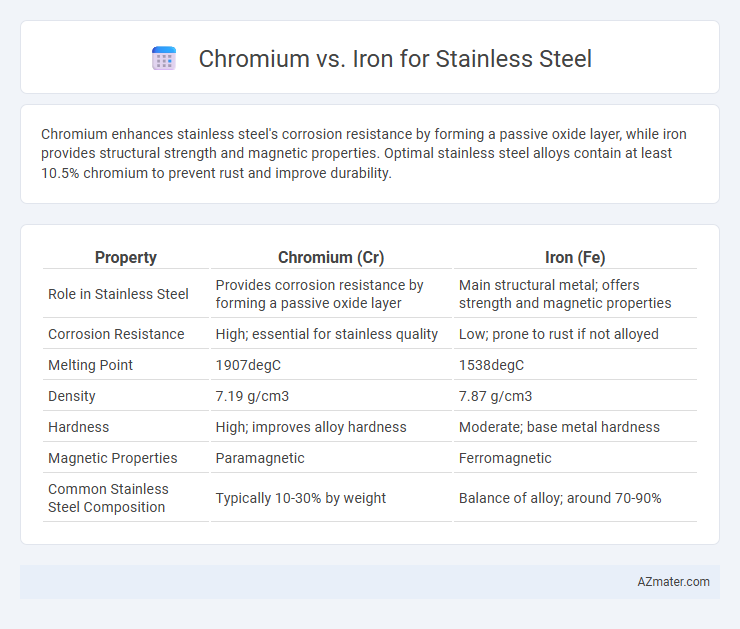
Chromium enhances stainless steel's corrosion resistance by forming a passive oxide layer, while iron provides structural strength and magnetic properties. Optimal stainless steel alloys contain at least 10.5% chromium to prevent rust and improve durability.
Table of Comparison
| Property | Chromium (Cr) | Iron (Fe) |
|---|---|---|
| Role in Stainless Steel | Provides corrosion resistance by forming a passive oxide layer | Main structural metal; offers strength and magnetic properties |
| Corrosion Resistance | High; essential for stainless quality | Low; prone to rust if not alloyed |
| Melting Point | 1907degC | 1538degC |
| Density | 7.19 g/cm3 | 7.87 g/cm3 |
| Hardness | High; improves alloy hardness | Moderate; base metal hardness |
| Magnetic Properties | Paramagnetic | Ferromagnetic |
| Common Stainless Steel Composition | Typically 10-30% by weight | Balance of alloy; around 70-90% |
Introduction to Chromium and Iron in Stainless Steel
Chromium and iron are essential components in stainless steel, each contributing distinct properties to the alloy. Chromium, typically added at 10.5% or more, forms a passive oxide layer that provides corrosion resistance and enhances durability. Iron serves as the base metal, offering structural strength and malleability essential for machining and fabrication.
Roles of Chromium and Iron in Stainless Steel Composition
Chromium plays a critical role in stainless steel by providing corrosion resistance through the formation of a thin, stable oxide layer on the surface, which protects the steel from rust and oxidation. Iron serves as the primary structural element, offering mechanical strength and ductility, forming the base matrix of the steel alloy. The combination of iron's mechanical properties and chromium's protective qualities results in stainless steel's durability and resistance to environmental degradation.
Chemical Properties: Chromium vs Iron
Chromium in stainless steel significantly enhances corrosion resistance due to its ability to form a stable, passive oxide layer that protects the alloy from oxidation and rust. Iron, while the primary base metal, is prone to oxidation and corrosion without the presence of chromium or other alloying elements. The chemical property contrast between chromium's high affinity for oxygen and iron's susceptibility to oxidation defines the superior durability and chemical stability of stainless steel.
Corrosion Resistance: Chromium’s Influence
Chromium significantly enhances corrosion resistance in stainless steel by forming a thin, stable oxide layer on the surface that prevents rust and degradation. Iron alone is prone to oxidation and corrosion, but the inclusion of at least 10.5% chromium creates a protective barrier that self-heals when damaged. This oxide layer is crucial for maintaining stainless steel's durability and resistance in harsh environments.
Strength and Durability: Iron’s Contribution
Chromium enhances stainless steel by providing corrosion resistance, while iron primarily contributes to its core strength and structural integrity. The iron content in stainless steel creates a robust metal matrix that supports load-bearing applications and resists deformation under stress. This combination of iron's mechanical strength with chromium's protective properties ensures stainless steel maintains durability in harsh environments.
Alloy Formation: How Chromium and Iron Interact
Chromium and iron form a substitutional alloy in stainless steel, where chromium atoms replace some iron atoms in the body-centered cubic lattice, enhancing corrosion resistance. The addition of at least 10.5% chromium creates a passive oxide layer that protects the steel from rust and oxidation. This interaction between chromium and iron is crucial for developing the alloy's durability and chemical stability in various environments.
Impact on Mechanical Properties
Chromium enhances the corrosion resistance and hardness of stainless steel by forming a passive oxide layer, significantly improving its mechanical strength and wear resistance. Iron serves as the primary structural matrix, providing ductility and toughness but lacks corrosion resistance on its own. The balance between chromium and iron content directly influences tensile strength, hardness, and the alloy's overall durability in various environmental conditions.
Cost Differences: Chromium vs Iron in Production
Chromium significantly increases the cost of stainless steel production compared to iron due to its higher market price and more complex extraction process. While iron serves as the base metal, chromium's addition enhances corrosion resistance but requires expensive alloying and refining techniques. The cost difference between chromium and iron directly impacts the overall price of stainless steel, making chromium content a key factor in production budgeting.
Environmental and Health Considerations
Chromium, essential in stainless steel for corrosion resistance, poses environmental concerns due to hexavalent chromium compounds that are toxic and carcinogenic during manufacturing and disposal. Iron, while abundant and less toxic, contributes to environmental impacts through mining and processing emissions but does not carry the same health risks as chromium. Sustainable stainless steel production prioritizes reducing chromium exposure and recycling to minimize ecological and human health hazards.
Choosing the Right Balance for Stainless Steel Applications
Choosing the right balance of chromium and iron in stainless steel is crucial for optimizing corrosion resistance and mechanical strength. Chromium content typically ranges from 10.5% to 30%, forming a passive oxide layer that protects the steel, while iron provides structural integrity and ductility. Adjusting the chromium-to-iron ratio allows for tailored properties in various applications, such as high corrosion environments favoring higher chromium levels and structural applications requiring more iron for toughness.
Infographic: Chromium vs Iron for Stainless Steel
 azmater.com
azmater.com