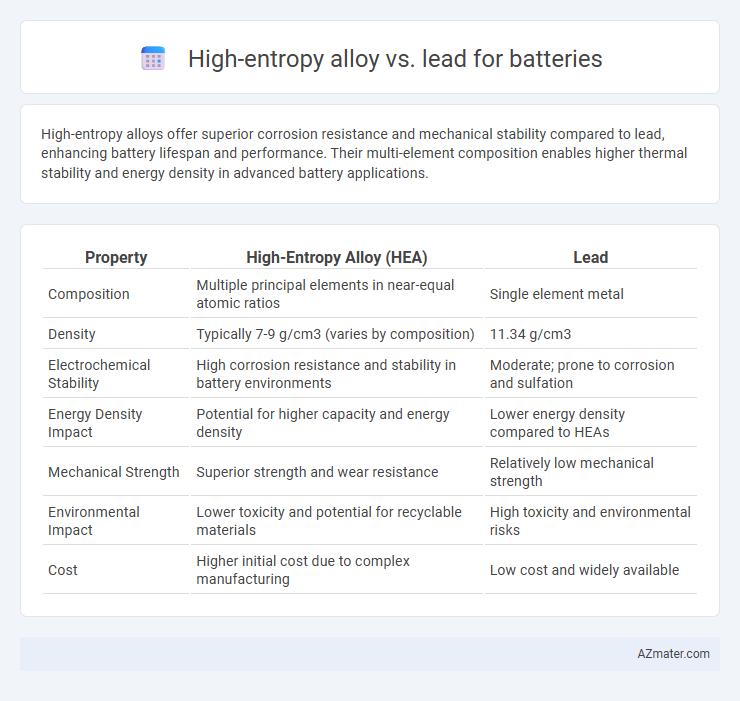
High-entropy alloys offer superior corrosion resistance and mechanical stability compared to lead, enhancing battery lifespan and performance. Their multi-element composition enables higher thermal stability and energy density in advanced battery applications.
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Lead |
|---|---|---|
| Composition | Multiple principal elements in near-equal atomic ratios | Single element metal |
| Density | Typically 7-9 g/cm3 (varies by composition) | 11.34 g/cm3 |
| Electrochemical Stability | High corrosion resistance and stability in battery environments | Moderate; prone to corrosion and sulfation |
| Energy Density Impact | Potential for higher capacity and energy density | Lower energy density compared to HEAs |
| Mechanical Strength | Superior strength and wear resistance | Relatively low mechanical strength |
| Environmental Impact | Lower toxicity and potential for recyclable materials | High toxicity and environmental risks |
| Cost | Higher initial cost due to complex manufacturing | Low cost and widely available |
Introduction to Battery Materials: High-Entropy Alloys vs Lead
High-entropy alloys (HEAs) exhibit superior mechanical strength, corrosion resistance, and thermal stability compared to traditional lead materials used in batteries, making them a promising alternative for advanced energy storage. Lead, commonly used in lead-acid batteries, provides reliable conductivity but suffers from weight and environmental concerns due to toxicity and limited cycle life. The innovative composition of HEAs, composed of multiple principal elements in near-equal atomic ratios, enables enhanced electrochemical performance and improved lifespan over conventional lead-based electrodes.
Composition and Structure: High-Entropy Alloys Explained
High-entropy alloys (HEAs) consist of five or more principal elements mixed in near-equimolar ratios, creating a complex, multi-component structure with high configurational entropy that stabilizes single-phase solid solutions. Unlike lead, which is a pure metal used traditionally in lead-acid batteries, HEAs exhibit a unique combination of elements such as nickel, cobalt, iron, chromium, and manganese, resulting in enhanced mechanical strength, corrosion resistance, and electrochemical stability. The intricate atomic arrangement and lattice distortion in HEAs contribute to improved battery performance by facilitating better ion diffusion and electrode durability compared to lead-based systems.
Traditional Role of Lead in Batteries
Lead has been the foundational material in battery technology due to its excellent electrochemical properties, high density, and cost-effectiveness that enable efficient energy storage and discharge in lead-acid batteries. These batteries dominate automotive starter systems and backup power supplies, offering reliable performance and recyclability. In contrast, high-entropy alloys are emerging materials with complex multi-element compositions that promise enhanced stability, corrosion resistance, and capacity for next-generation battery electrodes, potentially surpassing the limitations of traditional lead-based systems.
Electrochemical Performance Comparison
High-entropy alloys (HEAs) exhibit superior electrochemical performance compared to traditional lead in batteries, characterized by higher specific capacity and enhanced cycle stability. The multi-component composition of HEAs enables improved ion diffusion kinetics and reduced electrode degradation, leading to prolonged battery lifespan. In contrast, lead-based electrodes suffer from sulfation and limited charge-discharge efficiency, resulting in lower overall capacity and faster capacity fading during cycling.
Energy Density and Capacity: High-Entropy Alloy vs Lead
High-entropy alloys (HEAs) demonstrate significantly higher energy density and capacity compared to traditional lead-based batteries, enabling greater power storage within a smaller volume. HEAs benefit from their multi-element composition, which provides enhanced electrochemical stability and cycling performance, resulting in improved charge retention and lifespan. In contrast, lead-acid batteries typically suffer from lower energy density and faster capacity degradation, limiting their efficiency for modern, high-demand applications.
Cycle Life and Durability Analysis
High-entropy alloys (HEAs) demonstrate significantly enhanced cycle life and durability compared to traditional lead-based batteries, attributed to their multi-element composition that improves structural stability and resistance to degradation. Studies show HEA electrodes maintain capacity retention above 90% after over 1000 charge-discharge cycles, far surpassing the typical 300-500 cycles observed in lead-acid batteries. The superior corrosion resistance and mechanical robustness of HEAs contribute to prolonged battery lifespan and reliable performance under high-stress cycling conditions.
Safety and Environmental Impact Assessment
High-entropy alloys (HEAs) exhibit superior thermal stability and resistance to dendrite formation, significantly enhancing battery safety compared to traditional lead-based batteries, which suffer from corrosion and lead leakage risks. Environmental impact assessments reveal that HEAs reduce toxic metal contamination and improve recyclability, while lead batteries pose substantial hazards due to lead's neurotoxicity and challenges in disposal. Transitioning to HEA-based battery technology minimizes ecological footprint and health hazards associated with lead, aligning with sustainable energy storage goals.
Cost and Scalability Considerations
High-entropy alloys (HEAs) offer a promising alternative to lead in battery applications due to their potential for enhanced durability and electrochemical performance, although their current production costs remain significantly higher than those of lead-based materials. Scalability challenges stem from the complex synthesis processes required for HEAs, which involve precise control over multiple elemental compositions, limiting mass production capabilities compared to the well-established lead battery manufacturing infrastructure. Economies of scale and advances in fabrication technologies are crucial to reduce HEA costs and enable competitive scalability for broader adoption in energy storage systems.
Recent Advances in High-Entropy Alloys for Batteries
Recent advances in high-entropy alloys (HEAs) have demonstrated enhanced electrochemical stability, high capacity retention, and improved cycling performance in battery applications compared to traditional lead-based electrodes. Studies highlight HEAs' multi-element compositions that provide superior corrosion resistance and mechanical durability, enabling longer battery life and faster charge-discharge rates. Innovations in HEA synthesis and nanoscale structuring continue to optimize their conductivity and energy density, positioning them as promising alternatives to lead in next-generation energy storage technologies.
Future Prospects: High-Entropy Alloys as Lead Alternatives
High-entropy alloys (HEAs) demonstrate significant potential as sustainable alternatives to lead in battery technology due to their exceptional corrosion resistance, mechanical strength, and electrochemical stability. The unique multi-element composition of HEAs can enhance battery lifespan and energy density while reducing environmental impact associated with lead toxicity. Ongoing research emphasizes optimizing HEA formulations to achieve cost-effective, high-performance batteries that align with future energy storage demands and regulatory standards.
Infographic: High-entropy alloy vs Lead for Battery
 azmater.com
azmater.com