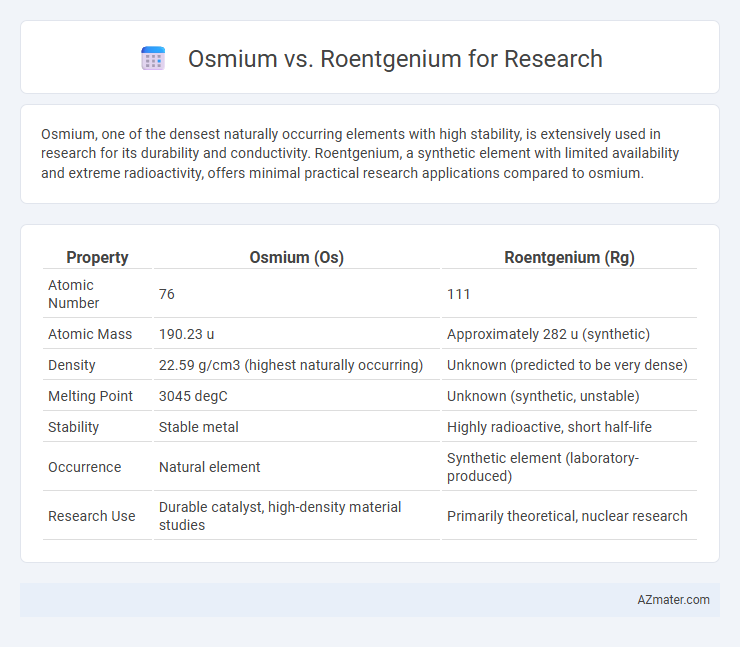
Osmium, one of the densest naturally occurring elements with high stability, is extensively used in research for its durability and conductivity. Roentgenium, a synthetic element with limited availability and extreme radioactivity, offers minimal practical research applications compared to osmium.
Table of Comparison
| Property | Osmium (Os) | Roentgenium (Rg) |
|---|---|---|
| Atomic Number | 76 | 111 |
| Atomic Mass | 190.23 u | Approximately 282 u (synthetic) |
| Density | 22.59 g/cm3 (highest naturally occurring) | Unknown (predicted to be very dense) |
| Melting Point | 3045 degC | Unknown (synthetic, unstable) |
| Stability | Stable metal | Highly radioactive, short half-life |
| Occurrence | Natural element | Synthetic element (laboratory-produced) |
| Research Use | Durable catalyst, high-density material studies | Primarily theoretical, nuclear research |
Introduction to Osmium and Roentgenium
Osmium, a dense transition metal with atomic number 76, is widely used in scientific research due to its hardness, high density (22.59 g/cm3), and resistance to corrosion, making it valuable for applications in material science and high-precision instruments. Roentgenium, element 111, is a synthetic, highly unstable superheavy element with a very short half-life, primarily studied through particle accelerator experiments to understand nuclear physics and the properties of transactinide elements. Research on osmium focuses on practical applications and physical properties, while roentgenium research is largely theoretical, aimed at expanding knowledge on the limits of the periodic table and nuclear stability.
Atomic Structure and Properties Comparison
Osmium, with atomic number 76, is a dense transition metal known for its high melting point and significant electron density, making it valuable for applications requiring extreme hardness and stability. Roentgenium, element 111, is a synthetic superheavy element with a transient half-life and limited experimental data, primarily studied for its predicted relativistic effects on electronic structure and potential deviations in chemical behavior. Comparative analysis of osmium's stable d-block configuration and roentgenium's relativistically influenced electron orbitals provides insights into atomic structure impacts on elemental properties in heavy metal research.
Elemental Rarity and Natural Occurrence
Osmium, a naturally occurring platinum-group metal, is one of the densest elements found in the Earth's crust with an abundance of approximately 0.0015 parts per million, making it rare but accessible for research purposes. Roentgenium, an artificially synthesized element with atomic number 111, does not occur naturally and is produced in minute quantities through particle accelerator experiments, significantly limiting its availability for practical research. The elemental rarity and natural occurrence of osmium present more feasible opportunities for extensive experimental studies compared to the transient and highly scarce roentgenium isotopes.
Historical Discovery and Naming
Osmium was discovered in 1803 by Smithson Tennant during the analysis of platinum ore and named for its dense, heavy properties derived from the Greek word "osme," meaning smell. Roentgenium, element 111, was first synthesized in 1994 by a team led by Peter Armbruster and Gottfried Munzenberg at the GSI Helmholtz Centre for Heavy Ion Research, named in honor of Wilhelm Conrad Roentgen, the discoverer of X-rays. Both elements' discoveries reflect advances in chemistry and physics, with osmium's natural occurrence contrasting roentgenium's synthetic origin in nuclear research.
Chemical Reactivity and Stability
Osmium exhibits high chemical stability with low reactivity due to its dense electron configuration and strong metallic bonding, making it valuable in catalysis and durable alloys. Roentgenium, an element with atomic number 111, remains largely uncharacterized due to its extreme radioactivity and short half-life, resulting in limited chemical data but predictions suggest higher reactivity typical of superheavy elements in group 11. Research on roentgenium primarily focuses on its synthesis and decay properties, while osmium's well-documented stability supports ongoing applications in chemical and material sciences.
Applications in Scientific Research
Osmium, with its high density and corrosion resistance, is extensively used in scientific research for creating durable electrodes and catalysts in electrochemical experiments. Roentgenium, a synthetic element with a very short half-life, remains primarily of theoretical interest and is limited to experimental nuclear physics studies involving superheavy element synthesis. The practical applications of Roentgenium in research are currently minimal due to its instability, whereas osmium's physical properties lend it significant utility in precise instrumentation and material science.
Safety and Handling Considerations
Osmium, with its high density and relative chemical stability, requires careful handling due to its toxic osmium tetroxide fumes, which pose significant health risks during research. Roentgenium, a synthetic element with a very short half-life and extremely limited availability, presents unique safety challenges, including intense radioactivity and the need for specialized containment to prevent radiation exposure. Research involving roentgenium demands advanced radiological protection protocols, while osmium handling prioritizes chemical safety and ventilation controls.
Cost and Availability for Laboratories
Osmium, a dense and rare transition metal, is more accessible and cost-effective for research laboratories compared to Roentgenium, a synthetic element with extremely limited availability and high production costs due to its radioactive nature and short half-life. The high expense and difficulty in obtaining Roentgenium restrict its use primarily to specialized nuclear research facilities with advanced particle accelerators. Osmium's relative abundance and established supply chains make it a practical choice for investigations requiring stable heavy metals.
Challenges in Experimental Usage
Osmium, with its high density and relative chemical stability, presents challenges in handling and precise measurement due to its brittleness and toxicity, complicating experimental setups. Roentgenium, a synthetic element with an extremely short half-life measured in milliseconds, imposes significant difficulties in experimental research, limiting its availability and hindering the study of its chemical properties. These constraints make osmium more accessible for practical experimentation, while roentgenium remains primarily a subject of transient nuclear research.
Future Prospects in Elemental Research
Osmium, known for its exceptional density and stability, serves as a crucial standard in high-precision measurement technologies and advanced catalysis research. Roentgenium, a superheavy synthetic element with limited isotopes and extreme instability, presents ongoing challenges but promises groundbreaking insights into nuclear physics and the island of stability theory. Future elemental research anticipates leveraging osmium's practical applications alongside exploratory studies of roentgenium's properties to expand understanding of superheavy elements and novel atomic behaviors.
Infographic: Osmium vs Roentgenium for Research
 azmater.com
azmater.com