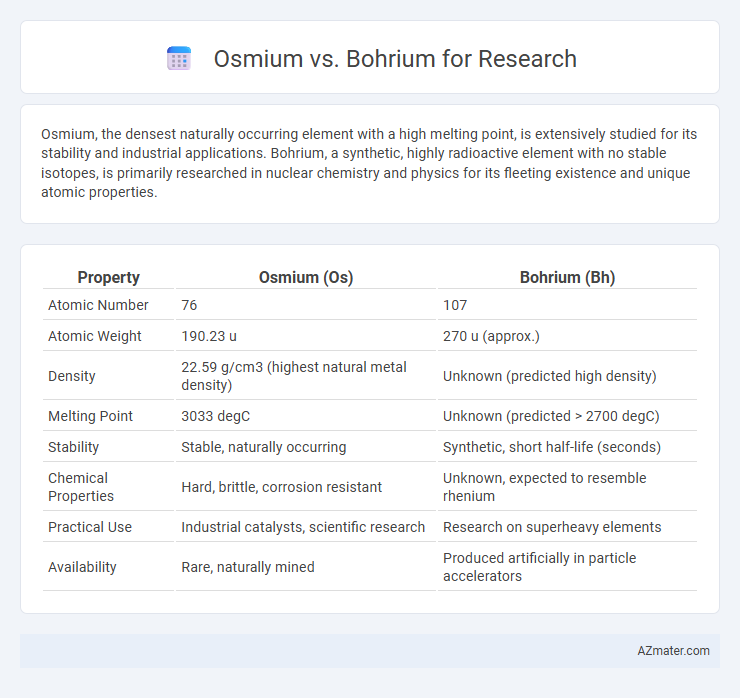
Osmium, the densest naturally occurring element with a high melting point, is extensively studied for its stability and industrial applications. Bohrium, a synthetic, highly radioactive element with no stable isotopes, is primarily researched in nuclear chemistry and physics for its fleeting existence and unique atomic properties.
Table of Comparison
| Property | Osmium (Os) | Bohrium (Bh) |
|---|---|---|
| Atomic Number | 76 | 107 |
| Atomic Weight | 190.23 u | 270 u (approx.) |
| Density | 22.59 g/cm3 (highest natural metal density) | Unknown (predicted high density) |
| Melting Point | 3033 degC | Unknown (predicted > 2700 degC) |
| Stability | Stable, naturally occurring | Synthetic, short half-life (seconds) |
| Chemical Properties | Hard, brittle, corrosion resistant | Unknown, expected to resemble rhenium |
| Practical Use | Industrial catalysts, scientific research | Research on superheavy elements |
| Availability | Rare, naturally mined | Produced artificially in particle accelerators |
Overview of Osmium and Bohrium
Osmium, a dense transition metal with atomic number 76, is renowned for its exceptional hardness and high melting point, making it valuable in industrial research and applications involving extreme conditions. Bohrium, element 107, is a synthetic, highly radioactive transactinide with limited practical use, primarily studied in nuclear chemistry research to understand heavy element behavior and stability. Research on Osmium focuses on its material properties and catalytic potential, while Bohrium investigations advance fundamental knowledge of superheavy element synthesis and decay characteristics.
Elemental Properties Comparison
Osmium, atomic number 76, is one of the densest naturally occurring elements with a high melting point of 3045degC and remarkable chemical stability in elemental form, making it suitable for high-pressure and wear-resistant applications in research. Bohrium, atomic number 107, is a synthetic, highly radioactive transactinide element with a very short half-life measured in seconds, which limits its practical study predominantly to theoretical and nuclear chemistry research. Comparing their elemental properties, osmium's stable isotopes and dense metallic nature contrast sharply with bohrium's transient existence and complex nuclear behavior, impacting their roles and methodologies in scientific investigation.
Historical Discovery and Nomenclature
Osmium, discovered in 1803 by Smithson Tennant, was named for its osme, meaning "smell," due to the distinct odor of its volatile oxide. Bohrium, synthesized in 1981 at the Gesellschaft fur Schwerionenforschung in Germany, honors physicist Niels Bohr, reflecting its status as a named transactinide element. The historical discovery and nomenclature of these elements highlight osmium's early identification in the platinum group metals and bohrium's connection to advanced nuclear research.
Natural Occurrence and Synthesis
Osmium is a naturally occurring element found in the Earth's crust with an average abundance of approximately 1 part per 100 million, making it one of the rarest elements but accessible for research involving natural isotopes and catalytic properties. Bohrium, on the other hand, is a synthetic element produced exclusively in particle accelerators through nuclear reactions involving heavy ion bombardment, with no stable or naturally occurring isotopes. The synthesis of bohrium enables the study of superheavy elements and their nuclear properties, while osmium's natural availability supports research in material science and catalysis.
Atomic Structure and Electron Configuration
Osmium (Os), with atomic number 76, possesses a stable electron configuration of [Xe] 4f14 5d6 6s2, making it suitable for studies in d-block transition metals and their unique catalytic properties. Bohrium (Bh), atomic number 107, is a synthetic element with an electron configuration approximated as [Rn] 5f14 6d5 7s2, reflecting its position in the 7th period of the periodic table and challenges in experimental research due to its short half-life and radioactive decay. Research comparing their atomic structures aids in understanding relativistic effects on electron behavior in heavy elements and the impact on chemical reactivity within the transactinide series.
Chemical Reactivity and Behavior
Osmium exhibits notable chemical reactivity characterized by its strong oxidation states, especially +8, enabling extensive compound formation such as osmium tetroxide, widely utilized in organic synthesis and microscopy. Bohrium, a synthetic superheavy element with limited experimental data, is predicted to behave similarly to its lighter homolog, rhenium, with expected oxidation states primarily around +7, but its chemical behavior remains largely theoretical due to its short half-life and production challenges. Research on osmium's stable and diverse chemistry contrasts with bohrium's speculative reactivity, highlighting osmium's practical significance in catalysis and material science versus bohrium's role in advancing nuclear and relativistic chemistry understanding.
Applications in Scientific Research
Osmium, with its exceptional density and catalytic properties, is extensively used in electron microscopy and as a catalyst in organic synthesis, enabling precise molecular transformations and advanced material studies. Bohrium, a synthetic element with a short half-life, is primarily utilized in nuclear chemistry research to investigate the behavior of heavy elements and the properties of transactinides, contributing to the understanding of nuclear reactions and element formation. Research involving osmium focuses on practical chemical applications and material science, while bohrium studies aim to expand knowledge of atomic structure and nuclear stability in superheavy elements.
Availability and Handling Challenges
Osmium, a dense transition metal with atomic number 76, is relatively more available and easier to handle compared to bohrium, a synthetic element with atomic number 107 that exists only in trace amounts produced in particle accelerators. The scarcity of bohrium, combined with its high radioactivity and short half-life, presents significant challenges for researchers in terms of safe handling and experimental stability. Osmium's availability allows for more practical laboratory use, though its toxicity and the volatility of its oxide require careful handling protocols.
Safety Precautions and Hazards
Osmium, a dense transition metal, requires careful handling due to its toxic osmium tetroxide, which poses inhalation and skin exposure hazards, necessitating the use of fume hoods and protective gloves in research settings. Bohrium, a synthetic element with a short half-life, presents significant radiological hazards, demanding remote handling techniques, specialized containment, and strict adherence to radiation safety protocols. Both elements compel rigorous safety measures to prevent chemical toxicity and radiological exposure risks, ensuring researcher protection during experimental procedures.
Future Prospects in Research
Osmium offers stable isotopes and exceptional density that make it ideal for advanced material science and catalysis research. Bohrium, a synthetic element with no stable isotopes, presents unique challenges yet promises breakthroughs in nuclear chemistry and superheavy element studies. Future research on bohrium focuses on synthesizing heavier elements and understanding relativistic effects, while osmium continues to drive innovations in high-pressure physics and nanotechnology.
Infographic: Osmium vs Bohrium for Research
 azmater.com
azmater.com