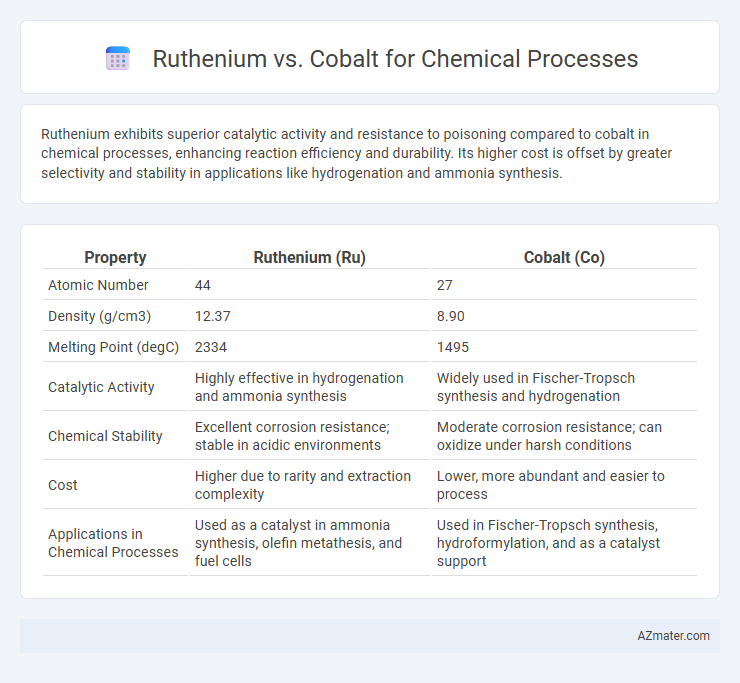
Ruthenium exhibits superior catalytic activity and resistance to poisoning compared to cobalt in chemical processes, enhancing reaction efficiency and durability. Its higher cost is offset by greater selectivity and stability in applications like hydrogenation and ammonia synthesis.
Table of Comparison
| Property | Ruthenium (Ru) | Cobalt (Co) |
|---|---|---|
| Atomic Number | 44 | 27 |
| Density (g/cm3) | 12.37 | 8.90 |
| Melting Point (degC) | 2334 | 1495 |
| Catalytic Activity | Highly effective in hydrogenation and ammonia synthesis | Widely used in Fischer-Tropsch synthesis and hydrogenation |
| Chemical Stability | Excellent corrosion resistance; stable in acidic environments | Moderate corrosion resistance; can oxidize under harsh conditions |
| Cost | Higher due to rarity and extraction complexity | Lower, more abundant and easier to process |
| Applications in Chemical Processes | Used as a catalyst in ammonia synthesis, olefin metathesis, and fuel cells | Used in Fischer-Tropsch synthesis, hydroformylation, and as a catalyst support |
Introduction to Ruthenium and Cobalt in Chemical Processes
Ruthenium and cobalt are transition metals widely utilized as catalysts in chemical processes due to their unique electronic and catalytic properties. Ruthenium exhibits exceptional activity in hydrogenation, oxidation, and ammonia synthesis reactions, often enhancing selectivity and stability under harsh reaction conditions. Cobalt is extensively employed in Fischer-Tropsch synthesis and hydroformylation, offering cost-effective catalytic performance and facilitating the production of hydrocarbons and aldehydes in industrial applications.
Chemical Properties: Ruthenium vs Cobalt
Ruthenium exhibits higher oxidation states ranging from +2 to +8, enabling versatile catalytic applications compared to cobalt's common oxidation states of +2 and +3. Ruthenium's greater resistance to corrosion and oxidation enhances its durability in harsh chemical environments unlike cobalt, which is more prone to oxidation. The unique ability of ruthenium to form stable complexes and facilitate redox reactions makes it superior in catalytic processes such as ammonia synthesis and hydrogenation when compared to cobalt.
Catalytic Efficiency: Comparing Ruthenium and Cobalt
Ruthenium exhibits superior catalytic efficiency compared to cobalt in hydrogenation and ammonia synthesis due to its higher activation energy and selectivity. Ruthenium catalysts demonstrate enhanced turnover frequency and resistance to poisoning, making them more effective in harsh chemical process environments. Cobalt offers cost advantages but typically requires higher temperatures and pressures to achieve comparable reaction rates.
Industrial Applications of Ruthenium and Cobalt
Ruthenium and cobalt play pivotal roles in chemical processes, with ruthenium prized for its exceptional catalytic properties in industrial hydrogenation, ammonia synthesis, and organometallic chemistry, boosting reaction efficiency and selectivity. Cobalt is widely utilized in Fischer-Tropsch synthesis and hydroformylation, catalyzing the production of synthetic fuels and aldehydes with industrial scalability. Both metals are critical in refining technologies, with ruthenium enhancing catalytic converters while cobalt is integral to battery electrodes and superalloys, underscoring their complementary industrial applications.
Cost Analysis: Ruthenium vs Cobalt
Ruthenium offers superior catalytic efficiency but comes at a significantly higher cost compared to cobalt, which remains a more economical choice for large-scale chemical processes. The price per gram of ruthenium can be several times that of cobalt, impacting the overall budget, especially in continuous industrial applications. Despite the higher expense, ruthenium's durability and enhanced reaction specificity may reduce operational costs by increasing catalyst longevity and process throughput.
Environmental Impact and Sustainability
Ruthenium offers higher catalytic efficiency and selectivity in chemical processes, reducing energy consumption and waste generation compared to cobalt. Cobalt mining poses significant environmental challenges, including habitat destruction and toxic byproduct release, while ruthenium, often recovered from platinum group metals recycling, supports circular economy principles. Sustainable chemical processes favor ruthenium catalysts due to their longer lifespan and lower ecological footprint, promoting greener industrial practices.
Availability and Sourcing Challenges
Ruthenium, a rare platinum-group metal, faces significant availability constraints due to limited extraction primarily from South African and Russian mines, making its sourcing complex and supply volatile. In contrast, cobalt is more abundant, largely sourced from the Democratic Republic of Congo, but its supply chain is fraught with ethical and political challenges impacting stable procurement. Both metals require careful consideration of geopolitical factors and market demand fluctuations when used in chemical process applications.
Safety and Handling Considerations
Ruthenium and cobalt, both transition metals used in chemical processes, differ significantly in safety and handling considerations. Ruthenium compounds may pose risks of toxicity and require careful management of dust and fumes, while cobalt exposure is linked to respiratory issues and allergic reactions, necessitating strict personal protective equipment measures. Safe storage and handling protocols emphasize proper ventilation, use of gloves, and minimizing dust inhalation for both metals to prevent health hazards in industrial environments.
Current Trends in Catalyst Research
Ruthenium exhibits superior catalytic activity and selectivity in hydrogenation and ammonia synthesis compared to cobalt, making it a preferred choice in high-performance catalyst development. Current trends emphasize ruthenium's ability to facilitate reactions under milder conditions while maintaining durability and resistance to deactivation. Research is increasingly focused on optimizing ruthenium-based catalysts through nanoengineering and alloying to reduce costs and enhance catalytic efficiency relative to cobalt counterparts.
Conclusion: Choosing Between Ruthenium and Cobalt
Ruthenium offers superior catalytic performance in chemical processes requiring high selectivity and resistance to harsh conditions, making it ideal for fine chemical synthesis and hydrogenation reactions. Cobalt provides a cost-effective alternative with good activity in Fischer-Tropsch synthesis and hydrogenation, suitable for large-scale industrial applications. Selecting between ruthenium and cobalt depends on balancing performance requirements, catalyst stability, and budget constraints specific to the chemical process.
Infographic: Ruthenium vs Cobalt for Chemical Process
 azmater.com
azmater.com