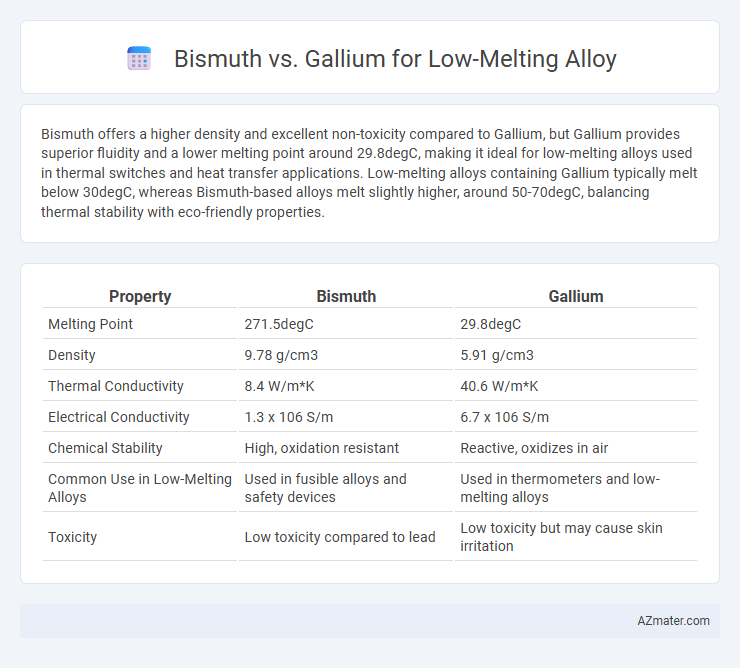
Bismuth offers a higher density and excellent non-toxicity compared to Gallium, but Gallium provides superior fluidity and a lower melting point around 29.8degC, making it ideal for low-melting alloys used in thermal switches and heat transfer applications. Low-melting alloys containing Gallium typically melt below 30degC, whereas Bismuth-based alloys melt slightly higher, around 50-70degC, balancing thermal stability with eco-friendly properties.
Table of Comparison
| Property | Bismuth | Gallium |
|---|---|---|
| Melting Point | 271.5degC | 29.8degC |
| Density | 9.78 g/cm3 | 5.91 g/cm3 |
| Thermal Conductivity | 8.4 W/m*K | 40.6 W/m*K |
| Electrical Conductivity | 1.3 x 106 S/m | 6.7 x 106 S/m |
| Chemical Stability | High, oxidation resistant | Reactive, oxidizes in air |
| Common Use in Low-Melting Alloys | Used in fusible alloys and safety devices | Used in thermometers and low-melting alloys |
| Toxicity | Low toxicity compared to lead | Low toxicity but may cause skin irritation |
Introduction to Low-Melting Alloys
Low-melting alloys are critical in applications requiring precise temperature control, with Bismuth and Gallium being key components due to their exceptionally low melting points. Bismuth-based alloys typically melt around 70degC, offering stability and non-toxicity, while Gallium alloys melt near 30degC and provide superior thermal conductivity and corrosive resistance. These characteristics influence selection criteria in electronics, thermal interface materials, and fusible plugs, balancing melting point, mechanical strength, and environmental safety.
Key Properties of Bismuth
Bismuth exhibits a low melting point of 271.5degC and a high density of 9.78 g/cm3, making it ideal for low-melting alloys used in fire detection and fusible plugs. Its non-toxicity and excellent dimensional stability upon solidification differentiate it from other metals like gallium, which melts at 29.76degC and tends to be more reactive and corrosive. Bismuth alloys demonstrate superior brittleness and thermal expansion control, enhancing reliability in precision casting and soldering applications.
Key Properties of Gallium
Gallium exhibits a low melting point of approximately 29.76degC, making it ideal for low-melting alloys that require liquid phase formation near room temperature. Its excellent thermal conductivity and low toxicity give it an advantage over Bismuth in applications such as thermal interface materials and fusible alloys. Gallium also demonstrates superior wettability with metals, enhancing its effectiveness in soldering and electronic component fabrication.
Melting Points: Bismuth vs Gallium
Bismuth has a melting point of 271.5degC, significantly higher than Gallium's melting point of approximately 29.8degC, making Gallium ideal for applications requiring near-room-temperature melting alloys. Bismuth's higher melting point suits alloys needing thermal stability above 200degC, while Gallium's low melting point enables use in thermally sensitive environments and low-temperature fusible alloys. The stark contrast in melting points between Bismuth and Gallium is critical for selecting appropriate low-melting alloys based on operational temperatures.
Alloying Behavior and Compatibility
Bismuth and gallium exhibit distinct alloying behaviors influencing their application in low-melting alloys. Bismuth forms eutectic alloys with metals such as tin and lead, characterized by low melting points and good thermal conductivity, making it suitable for fusible alloys with predictable melting ranges. Gallium, with its ability to alloy with aluminum, zinc, and indium, offers exceptional wettability and ductility but may cause embrittlement in some metal matrices, requiring careful compatibility assessment for reliable low-melting alloy formulations.
Safety and Environmental Considerations
Bismuth-based low-melting alloys offer superior safety due to their non-toxic nature and environmental friendliness, making them ideal for applications requiring minimal ecological impact. Gallium alloys, while useful for low melting points, pose potential safety risks as gallium can cause skin irritation and is considered less environmentally benign. Choosing bismuth alloys minimizes hazardous waste concerns and ensures safer handling in manufacturing and disposal processes.
Applications in Industry
Bismuth-based low-melting alloys are widely used in fire safety devices and metal casting due to their non-toxic and environmentally friendly properties, while gallium-based alloys excel in electronics for thermal interface materials and flexible circuits owing to their superior electrical conductivity and wettability. The industry favors bismuth alloys for precision casting and fusible plugs because of their stable melting points and ease of handling, whereas gallium alloys are preferred in advanced cooling systems and soft robotics for their liquid metal state near room temperature. Both metals enable innovations in soldering and thermal management, but gallium's unique ability to alloy with aluminum makes it critical for aerospace and semiconductor applications.
Cost and Availability Comparison
Bismuth typically costs more than gallium, largely due to its lower abundance and higher extraction costs, making it less economical for large-scale low-melting alloy production. Gallium is more readily available as a byproduct of aluminum and zinc refining, which supports its relatively stable market price and easier procurement. The cost-effectiveness and availability of gallium make it a preferred choice over bismuth in applications requiring frequent or large-volume alloy usage.
Performance in Common Alloys
Bismuth-based low-melting alloys typically exhibit excellent thermal expansion control and high density, enhancing dimensional stability in precision castings, whereas gallium alloys offer superior fluidity and lower melting points around 30degC to 50degC, ideal for applications requiring minimal heat exposure. Common bismuth alloys like Bi-Sn-Pb and Bi-Cd-Sn demonstrate better strength and corrosion resistance compared to gallium-tin alloys, which prioritize non-toxicity and ease of melting but often sacrifice mechanical robustness. Performance evaluations show that bismuth alloys outperform gallium in load-bearing and thermal cycling scenarios, while gallium alloys excel in electronics cooling and rapid prototyping due to their unique melting characteristics and malleability.
Choosing the Best Metal for Your Alloy
Bismuth offers a low melting point around 271degC and expands upon solidification, making it ideal for applications requiring minimal thermal stress and precise casting. Gallium melts near 30degC and remains liquid just above room temperature, providing unique advantages for low-temperature soldering and thermally sensitive processes. When selecting the best metal for your low-melting alloy, consider Bismuth's structural stability and non-toxicity versus Gallium's exceptional fluidity and metal compatibility for optimal performance.
Infographic: Bismuth vs Gallium for Low-melting alloy
 azmater.com
azmater.com