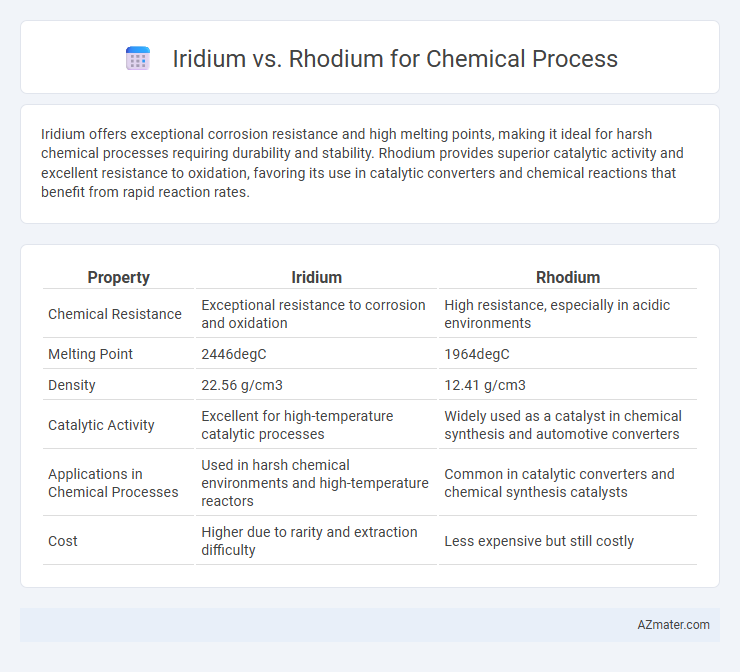
Iridium offers exceptional corrosion resistance and high melting points, making it ideal for harsh chemical processes requiring durability and stability. Rhodium provides superior catalytic activity and excellent resistance to oxidation, favoring its use in catalytic converters and chemical reactions that benefit from rapid reaction rates.
Table of Comparison
| Property | Iridium | Rhodium |
|---|---|---|
| Chemical Resistance | Exceptional resistance to corrosion and oxidation | High resistance, especially in acidic environments |
| Melting Point | 2446degC | 1964degC |
| Density | 22.56 g/cm3 | 12.41 g/cm3 |
| Catalytic Activity | Excellent for high-temperature catalytic processes | Widely used as a catalyst in chemical synthesis and automotive converters |
| Applications in Chemical Processes | Used in harsh chemical environments and high-temperature reactors | Common in catalytic converters and chemical synthesis catalysts |
| Cost | Higher due to rarity and extraction difficulty | Less expensive but still costly |
Introduction to Iridium and Rhodium
Iridium and rhodium are rare, precious platinum-group metals known for their exceptional catalytic properties in chemical processes. Iridium excels in high-temperature corrosion resistance and is often used in oxidation and hydrogenation reactions due to its durability and stability. Rhodium is highly valued for its superior catalytic activity in automotive catalytic converters and selective hydrogenation, making it essential for refining and chemical synthesis applications.
Chemical Properties Comparison
Iridium exhibits exceptional resistance to corrosion and high oxidation resistance, making it ideal for catalytic processes involving harsh chemical environments. Rhodium demonstrates superior catalytic activity in hydrogenation and oxidation reactions due to its high surface reactivity and excellent thermal stability. Both metals feature high melting points and strong chemical inertness, but rhodium's enhanced catalytic efficiency makes it preferred in industrial chemical processes requiring selective oxidation or hydrogenation.
Catalytic Activity in Chemical Processes
Iridium exhibits exceptional catalytic activity in chemical processes involving hydrogenation and oxidation reactions due to its high stability and resistance to deactivation under harsh conditions. Rhodium is renowned for its superior catalytic performance in carbon-carbon coupling and hydroformylation reactions, offering higher selectivity and turnover frequencies. The choice between iridium and rhodium catalysts depends on the specific reaction mechanism, temperature tolerance, and desired product yield in industrial applications.
Industrial Applications of Iridium vs Rhodium
Iridium excels in chemical processes requiring high corrosion resistance and stability at extreme temperatures, making it ideal for use in high-temperature crucibles and catalyst supports in industrial applications. Rhodium is favored for its exceptional catalytic properties, particularly in automotive catalytic converters and chemical synthesis reactions involving hydrogenation and oxidation. Industrially, iridium is preferred for durable electrodes and crucibles in chlor-alkali production, while rhodium dominates in refining processes due to its superior activity in catalytic converters and nitric acid production.
Cost and Market Availability
Iridium and rhodium are both valuable catalysts in chemical processes, with iridium generally exhibiting higher resistance to corrosion and oxidation but at a significantly higher cost, often exceeding $4,000 per ounce compared to rhodium's market price, which fluctuates around $10,000 per ounce due to its rarity and industrial demand. Rhodium's limited availability, with annual global production around 30 metric tons, contributes to its price volatility and challenges in sourcing, while iridium's relatively stable but low production volume, approximately 7 metric tons per year, affects its accessibility for large-scale applications. Cost efficiency and market availability make rhodium preferred for high-performance catalytic converters despite higher prices, whereas iridium is chosen for niche chemical processes requiring extreme durability.
Environmental Impact and Sustainability
Iridium and rhodium, both platinum-group metals, differ significantly in environmental impact and sustainability during chemical processing; iridium exhibits higher resistance to corrosion, leading to longer catalyst life and reduced material waste. Rhodium's catalytic efficiency in automotive emissions control reduces harmful gases but involves energy-intensive extraction processes that raise its environmental footprint. Selecting iridium for chemical processes enhances sustainability by lowering catalyst replacement frequency, whereas rhodium's application balances immediate emission reductions with long-term environmental costs.
Durability and Resistance to Corrosion
Iridium exhibits exceptional durability and resistance to corrosion, making it highly suitable for harsh chemical processes involving strong acids and oxidizing environments. Rhodium also offers excellent corrosion resistance but tends to be softer and less durable under prolonged high-temperature or highly aggressive chemical conditions compared to iridium. The superior hardness and stability of iridium enable longer catalyst life and enhanced performance in demanding industrial chemical applications.
Safety and Handling Considerations
Iridium and rhodium are both noble metals used as catalysts in chemical processes, but iridium exhibits higher corrosion resistance, making it safer for harsh chemical environments. Rhodium's lower melting point and greater brittleness require careful handling to prevent physical damage and exposure risks during catalytic reactions. Both metals necessitate protective measures due to their high cost and potential toxicity when in fine particulate or compound forms, emphasizing the importance of controlled ventilation and personal protective equipment in laboratory and industrial settings.
Recent Advances and Innovations
Iridium and rhodium are critical catalysts in chemical processes due to their exceptional stability and activity, with recent advances enhancing their efficiency in carbon-carbon bond formation and hydrogenation reactions. Innovations include the development of iridium-based pincer complexes that enable selective C-H activation under mild conditions, while rhodium catalysts have seen improvements in enantioselective hydroformylation, boosting yield and selectivity. Advanced nanostructuring techniques and ligand design have further optimized both metals for sustainable catalysis in pharmaceutical and fine chemical manufacturing.
Choosing the Right Metal for Chemical Processes
Iridium offers exceptional corrosion resistance and high melting points, making it ideal for harsh chemical environments and high-temperature catalysis. Rhodium excels in catalytic applications, particularly in hydrogenation and catalytic converters, due to its superior activity and selectivity. Choosing the right metal depends on specific reaction conditions, such as temperature tolerance, chemical stability, and catalytic efficiency required for the process.
Infographic: Iridium vs Rhodium for Chemical Process
 azmater.com
azmater.com