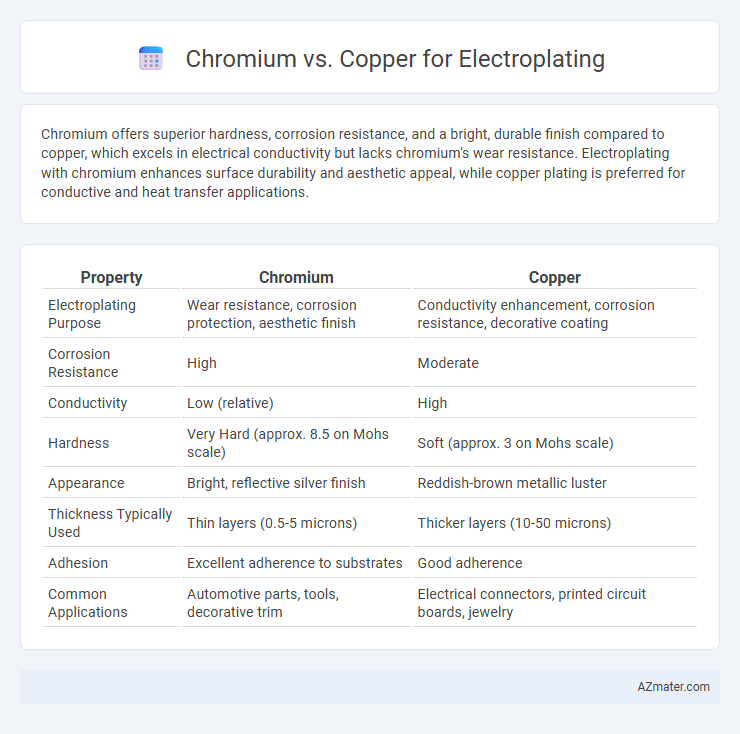
Chromium offers superior hardness, corrosion resistance, and a bright, durable finish compared to copper, which excels in electrical conductivity but lacks chromium's wear resistance. Electroplating with chromium enhances surface durability and aesthetic appeal, while copper plating is preferred for conductive and heat transfer applications.
Table of Comparison
| Property | Chromium | Copper |
|---|---|---|
| Electroplating Purpose | Wear resistance, corrosion protection, aesthetic finish | Conductivity enhancement, corrosion resistance, decorative coating |
| Corrosion Resistance | High | Moderate |
| Conductivity | Low (relative) | High |
| Hardness | Very Hard (approx. 8.5 on Mohs scale) | Soft (approx. 3 on Mohs scale) |
| Appearance | Bright, reflective silver finish | Reddish-brown metallic luster |
| Thickness Typically Used | Thin layers (0.5-5 microns) | Thicker layers (10-50 microns) |
| Adhesion | Excellent adherence to substrates | Good adherence |
| Common Applications | Automotive parts, tools, decorative trim | Electrical connectors, printed circuit boards, jewelry |
Introduction to Electroplating
Electroplating involves depositing a metal layer onto a substrate using an electric current, enhancing corrosion resistance and aesthetic appeal. Chromium and copper are common electroplating metals, with chromium providing a hard, shiny, and wear-resistant finish, while copper offers excellent conductivity and adhesion properties. Understanding the distinct electrochemical properties of chromium and copper is essential for selecting the appropriate plating metal based on application requirements.
Overview of Chromium Electroplating
Chromium electroplating provides a hard, corrosion-resistant, and aesthetically appealing surface commonly used in automotive and industrial applications. It involves depositing a thin layer of chromium onto a metal substrate through electrochemical reduction from a chromic acid solution. The process enhances wear resistance, reduces friction, and improves surface hardness compared to copper electroplating.
Overview of Copper Electroplating
Copper electroplating involves depositing a thin layer of copper onto a conductive surface using an electrolytic cell, enhancing corrosion resistance, electrical conductivity, and solderability. This process utilizes copper sulfate and sulfuric acid solutions, producing a smooth, uniform coating ideal for printed circuit boards and decorative finishes. Compared to chromium plating, copper electroplating offers superior electrical conductivity and excellent adhesion properties but provides less hardness and wear resistance.
Key Differences Between Chromium and Copper Plating
Chromium plating offers superior hardness and corrosion resistance compared to copper plating, making it ideal for wear-resistant and decorative surfaces. Copper plating excels in electrical conductivity and provides excellent adhesion for subsequent metal layers, often serving as a base layer in multi-step plating processes. The distinct surface finishes and thickness options between chromium's bright, reflective coating and copper's softer, malleable layer highlight their specialized applications in manufacturing and electronics.
Surface Finish and Appearance Comparison
Chromium electroplating provides a bright, mirror-like finish with excellent corrosion resistance, making it ideal for decorative applications requiring high luster and durability. Copper electroplating offers a warm, reddish surface with good conductivity and smoothness but lacks the reflective shine and hardness of chromium coatings. The choice between chromium and copper depends largely on the desired aesthetic, with chromium favored for a polished, metallic sheen and copper chosen for its natural, softer appearance.
Durability and Corrosion Resistance
Chromium electroplating offers superior durability and exceptional corrosion resistance compared to copper, making it ideal for applications requiring long-lasting protection against wear and environmental damage. Copper plating, while providing good electrical conductivity and aesthetic appeal, tends to be less resistant to corrosion and scratches. The hard, dense layer of chromium effectively shields underlying materials from oxidation and mechanical degradation, enhancing the lifespan of electroplated components.
Cost and Economic Considerations
Chromium electroplating typically incurs higher costs than copper due to expensive raw materials and complex processing requirements, influencing overall project budgets. Copper plating offers cost-effective advantages with cheaper materials and faster application rates, making it ideal for large-scale or budget-sensitive projects. Economic considerations must balance chromium's superior corrosion resistance and aesthetic appeal against copper's affordability and conductivity benefits.
Typical Applications of Chromium vs Copper
Chromium electroplating is commonly used in automotive parts, aerospace components, and industrial machinery for its hardness, corrosion resistance, and aesthetic mirror finish. Copper electroplating serves as an excellent conductive layer in electrical connectors, printed circuit boards, and decorative items due to its superior electrical conductivity and strong adhesion properties. Both metals are essential in electroplating for enhancing surface characteristics depending on whether durability or electrical performance is the priority.
Environmental and Safety Concerns
Chromium electroplating involves the use of hexavalent chromium, a highly toxic and carcinogenic substance that poses significant environmental hazards and strict regulatory compliance challenges due to its hazardous waste disposal requirements. Copper electroplating, while less toxic, releases copper ions into wastewater which can be harmful to aquatic life, necessitating effective wastewater treatment to prevent environmental contamination. Both processes require stringent safety measures and environmental controls to mitigate health risks and minimize ecological impact.
Choosing the Right Metal for Electroplating
Chromium offers superior hardness, corrosion resistance, and a bright, reflective finish, making it ideal for decorative and protective electroplating applications. Copper provides excellent electrical conductivity, ductility, and corrosion resistance, often serving as an undercoat to improve adhesion for subsequent plating layers. Selecting between chromium and copper depends on the functional requirements, with chromium best for wear resistance and aesthetics, while copper excels in conductivity and as a base layer in multi-metal plating processes.
Infographic: Chromium vs Copper for Electroplating
 azmater.com
azmater.com