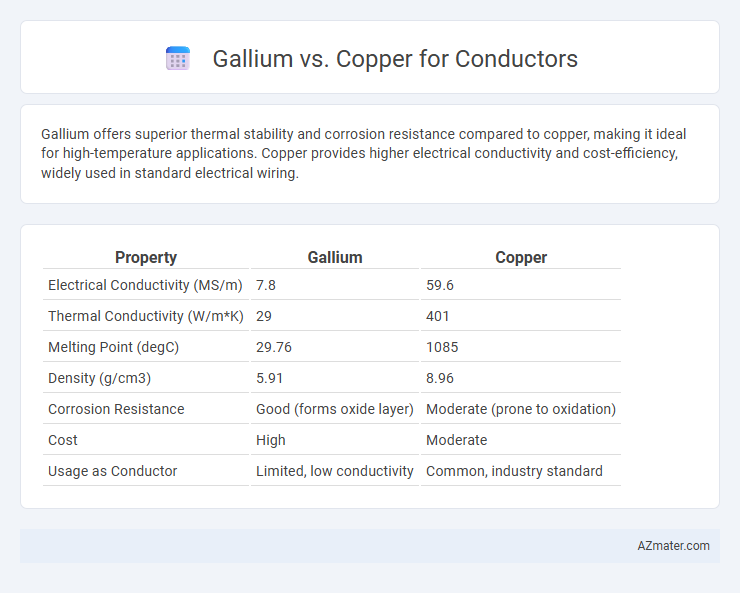
Gallium offers superior thermal stability and corrosion resistance compared to copper, making it ideal for high-temperature applications. Copper provides higher electrical conductivity and cost-efficiency, widely used in standard electrical wiring.
Table of Comparison
| Property | Gallium | Copper |
|---|---|---|
| Electrical Conductivity (MS/m) | 7.8 | 59.6 |
| Thermal Conductivity (W/m*K) | 29 | 401 |
| Melting Point (degC) | 29.76 | 1085 |
| Density (g/cm3) | 5.91 | 8.96 |
| Corrosion Resistance | Good (forms oxide layer) | Moderate (prone to oxidation) |
| Cost | High | Moderate |
| Usage as Conductor | Limited, low conductivity | Common, industry standard |
Introduction to Gallium and Copper as Conductors
Gallium and copper exhibit distinct electrical conductivity properties, with copper being a widely used conductor in electrical wiring due to its high conductivity of approximately 5.96 x 10^7 S/m and excellent durability. Gallium, a post-transition metal with a conductivity around 7.14 x 10^6 S/m, offers unique applications in flexible electronics and semiconductors due to its low melting point of about 29.76degC and liquid state near room temperature. While copper remains the standard choice for power transmission and electronic circuitry, gallium's emerging role lies in niche areas requiring pliable and thermally stable conductive materials.
Electrical Conductivity Comparison: Gallium vs Copper
Copper exhibits significantly higher electrical conductivity than gallium, with copper's conductivity approximately 5.96 x 10^7 S/m compared to gallium's much lower conductivity near 3.7 x 10^6 S/m. This makes copper the preferred material for electrical conductors due to its efficient electron flow and minimal resistive losses. Despite gallium's unique low melting point and ability to form liquid alloys, copper remains superior for most electrical wiring and power transmission applications.
Thermal Conductivity Differences
Gallium exhibits a thermal conductivity of approximately 29 W/m*K, significantly lower than copper's thermal conductivity, which is about 401 W/m*K, making copper far more efficient in heat dissipation. This substantial difference means copper is preferred in applications requiring rapid heat transfer and minimal thermal resistance, such as electrical wiring and heat sinks. Gallium's lower thermal conductivity limits its use as a conductor where thermal management is critical despite its unique liquid properties at near-room temperature.
Material Abundance and Cost Analysis
Copper remains the dominant conductor material due to its high abundance and relatively low cost, with global reserves estimated around 870 million metric tons and widespread mining infrastructure supporting its affordability. Gallium, in contrast, is a rare element with annual global production of about 400 metric tons, predominantly as a byproduct of aluminum and zinc refining, resulting in significantly higher costs per kilogram. The scarcity and extraction complexity of gallium drive costs up, making copper the more economically viable option for large-scale electrical conductors despite gallium's superior conductivity properties in specific applications.
Mechanical Properties and Durability
Gallium exhibits a lower tensile strength and hardness compared to copper, making copper more suitable for applications requiring mechanical robustness and resistance to deformation. Copper's excellent ductility and fatigue resistance contribute to its superior durability under cyclic mechanical stresses, whereas gallium's softness and brittleness limit its structural integrity in conductive roles. The stable microstructure of copper ensures long-term performance and corrosion resistance, while gallium tends to oxidize and degrade faster in environmental exposure.
Corrosion Resistance and Stability
Gallium exhibits superior corrosion resistance compared to copper due to its ability to form a stable, protective oxide layer that prevents further oxidation and degradation in harsh environments. Copper, while an excellent conductor, is prone to oxidation and corrosion, especially in moist or acidic conditions, which can compromise its conductivity and structural stability over time. The chemical inertness and stable oxide film of gallium make it a more durable conductor in corrosive settings, enhancing long-term performance and reliability.
Application Suitability in Electronics
Gallium, with its low melting point and excellent conductivity, is primarily used in specialized electronics such as semiconductors and optoelectronic devices where precise thermal control is critical. Copper remains the standard conductor in most electronics due to its superior electrical conductivity, mechanical strength, and cost-effectiveness for wiring and circuit boards. Applications demanding high reliability and efficient current flow, like power distribution and printed circuit boards, overwhelmingly favor copper over gallium.
Environmental and Safety Considerations
Gallium offers a lower environmental impact during extraction compared to copper, as it is typically recovered as a byproduct of mining other metals, reducing the need for dedicated mining operations. Copper mining involves extensive land disruption, higher energy consumption, and greater greenhouse gas emissions, making its environmental footprint significantly larger. From a safety perspective, gallium is non-toxic and poses minimal health risks, whereas copper exposure can cause skin irritation and long-term health issues if proper handling protocols are not followed.
Future Trends in Conductor Materials
Gallium's promising low melting point and superior thermal conductivity position it as a potential game-changer in future conductor materials, especially for flexible and wearable electronics. Copper remains dominant due to its high electrical conductivity and cost-effectiveness but faces challenges in weight and corrosion resistance, driving research toward gallium-based alloys and composites. Emerging trends highlight the integration of gallium with nanomaterials to create lightweight, efficient conductors that could surpass traditional copper in next-generation electrical applications.
Conclusion: Choosing Between Gallium and Copper
Gallium offers superior flexibility and corrosion resistance compared to copper, making it ideal for applications requiring malleability and long-term stability. Copper excels in electrical conductivity and cost-effectiveness, remaining the preferred choice for most standard electrical wiring and power transmission tasks. Choosing between gallium and copper depends on balancing performance demands with budget constraints and specific environmental conditions.
Infographic: Gallium vs Copper for Conductor
 azmater.com
azmater.com