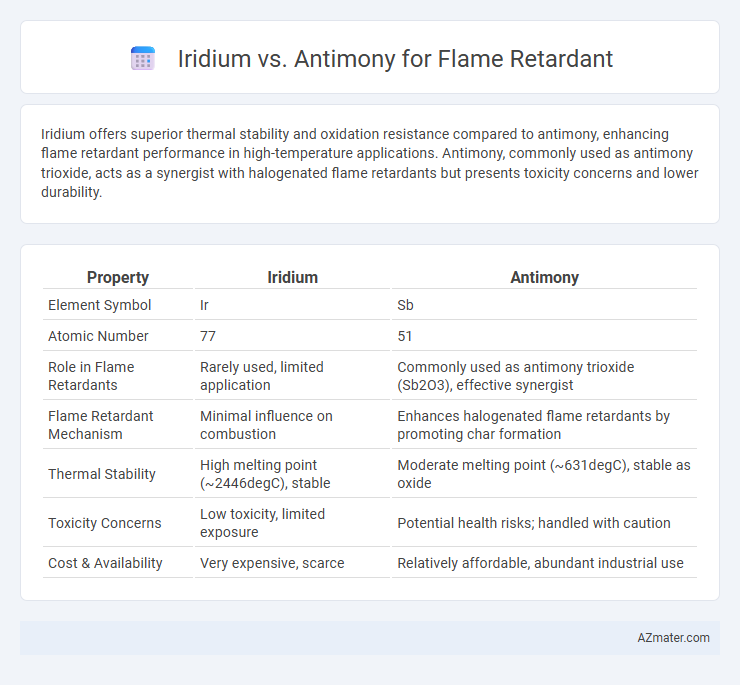
Iridium offers superior thermal stability and oxidation resistance compared to antimony, enhancing flame retardant performance in high-temperature applications. Antimony, commonly used as antimony trioxide, acts as a synergist with halogenated flame retardants but presents toxicity concerns and lower durability.
Table of Comparison
| Property | Iridium | Antimony |
|---|---|---|
| Element Symbol | Ir | Sb |
| Atomic Number | 77 | 51 |
| Role in Flame Retardants | Rarely used, limited application | Commonly used as antimony trioxide (Sb2O3), effective synergist |
| Flame Retardant Mechanism | Minimal influence on combustion | Enhances halogenated flame retardants by promoting char formation |
| Thermal Stability | High melting point (~2446degC), stable | Moderate melting point (~631degC), stable as oxide |
| Toxicity Concerns | Low toxicity, limited exposure | Potential health risks; handled with caution |
| Cost & Availability | Very expensive, scarce | Relatively affordable, abundant industrial use |
Introduction to Flame Retardants
Flame retardants are chemical substances applied to materials to inhibit or resist the spread of fire by interfering with combustion processes. Iridium and antimony are two elements utilized in flame retardant formulations, with antimony commonly used as antimony trioxide to enhance the effectiveness of halogenated flame retardants through synergistic mechanisms. Iridium, though less prevalent, offers potential in catalytic flame retardant systems due to its high thermal stability and ability to alter combustion pathways at a molecular level.
Overview of Iridium-Based Flame Retardants
Iridium-based flame retardants demonstrate exceptional thermal stability and catalytic properties that enhance flame retardant efficiency in polymers compared to traditional antimony compounds. These compounds facilitate char formation and inhibit combustion through advanced oxidative mechanisms, resulting in lower smoke and toxic gas emissions. Iridium's superior performance in high-temperature applications positions it as a promising substitute for antimony in environmentally sensitive flame retardant formulations.
Overview of Antimony-Based Flame Retardants
Antimony-based flame retardants, primarily antimony trioxide, serve as synergists in halogenated flame retardant systems by enhancing flame inhibition and smoke suppression. These compounds function by promoting char formation and releasing antimony halide radicals that quench free radicals in the flame, effectively reducing combustibility. Widely used in plastics, textiles, and coatings, antimony-based flame retardants are favored for their cost-efficiency and compatibility but pose environmental and health concerns compared to emerging alternatives like iridium-based systems.
Chemical Properties: Iridium vs Antimony
Iridium exhibits exceptional chemical stability and resistance to oxidation, making it highly durable under extreme temperatures, whereas antimony primarily acts as a synergist in flame retardants by enhancing char formation through its pentavalent oxidation state. Iridium's noble metal characteristics lead to minimal reactivity, while antimony compounds, especially antimony trioxide, promote flame retardancy by facilitating halogen radical scavenging and cross-linking in polymer matrices. This fundamental difference in chemical behavior defines iridium's limited role versus antimony's widespread application in flame retardant systems.
Flame Retardant Effectiveness Comparison
Iridium exhibits superior flame retardant effectiveness due to its high thermal stability and ability to form strong oxides that inhibit combustion, outperforming antimony which primarily acts through char formation and smoke suppression. Antimony compounds are more commonly used but often require synergists like halogenated flame retardants to achieve comparable performance to iridium-based systems. The enhanced efficiency of iridium results in lower loading levels needed for flame retardancy, reducing material weight and toxicity in finished products.
Environmental Impact: Sustainability and Toxicity
Iridium and antimony are used in flame retardants with distinct environmental impacts; iridium compounds tend to be less toxic but are rare and expensive, making their sustainability challenging. Antimony-based flame retardants are more common and cost-effective but pose significant environmental and health risks due to bioaccumulation and toxicity in aquatic systems. Sustainable flame retardant development increasingly favors alternatives to antimony because of these ecological and human health concerns.
Safety Concerns and Regulatory Status
Iridium is rarely used as a flame retardant due to its high cost and limited effectiveness, while antimony compounds, particularly antimony trioxide, are widely employed as synergists in halogenated flame retardants. Safety concerns regarding antimony involve its classification as a possible human carcinogen and potential toxicity through inhalation and skin exposure, prompting strict regulatory controls in regions such as the EU and the US. Regulatory agencies impose limits on antimony content and exposure levels, whereas iridium faces fewer regulatory restrictions due to its minimal application in flame retardancy.
Cost Analysis: Iridium vs Antimony
Iridium-based flame retardants are significantly more expensive than antimony counterparts due to iridium's rarity and complex extraction processes, leading to higher raw material and production costs. Antimony, available in larger quantities and produced through more established methods, offers a cost-effective solution with sufficient flame retardant properties for many industrial applications. When analyzing budgets, antimony stands out as the economical choice for flame retardant additives, whereas iridium may be reserved for specialized uses demanding superior performance despite its premium price.
Industrial Applications and Performance
Iridium offers exceptional thermal stability and corrosion resistance, making it suitable for high-performance flame retardant applications in aerospace and electronics industries where durability under extreme conditions is critical. Antimony, widely used as antimony trioxide, enhances the effectiveness of halogenated flame retardants by promoting char formation and smoke suppression, primarily in plastics, textiles, and construction materials. Industrial applications favor antimony for cost-efficient large-scale production, while iridium's superior performance justifies its use in specialized, high-value environments requiring prolonged flame resistance.
Future Trends in Flame Retardant Materials
Iridium and antimony are both critical elements used in flame retardant materials, with iridium gaining attention for its potential in high-performance, eco-friendly applications due to its catalytic properties and stability at high temperatures. Future trends highlight a shift towards iridium-based compounds to replace the more toxic and environmentally persistent antimony, promoting safer and more sustainable flame retardant solutions. Research is increasingly focused on enhancing iridium's efficiency in flame retardancy while minimizing environmental impact, driving innovation in advanced polymer composites and electronic device coatings.
Infographic: Iridium vs Antimony for Flame Retardant
 azmater.com
azmater.com