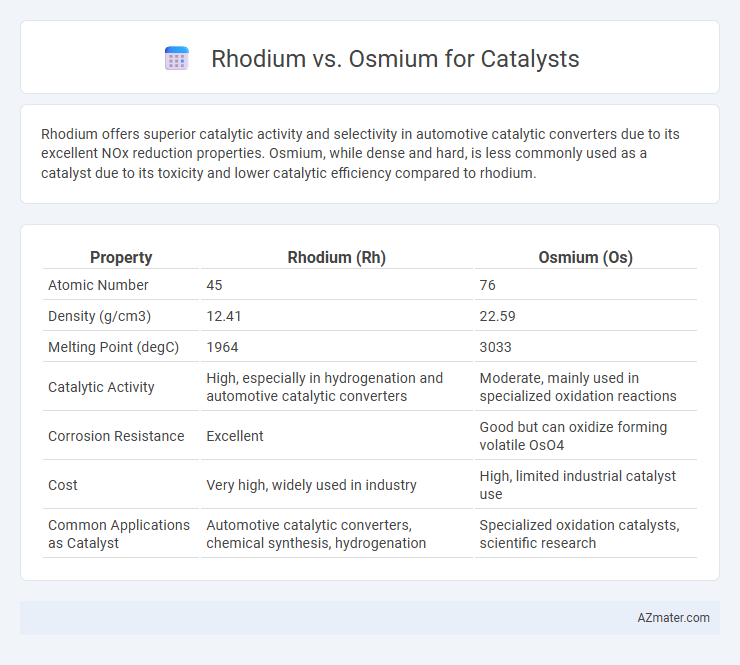
Rhodium offers superior catalytic activity and selectivity in automotive catalytic converters due to its excellent NOx reduction properties. Osmium, while dense and hard, is less commonly used as a catalyst due to its toxicity and lower catalytic efficiency compared to rhodium.
Table of Comparison
| Property | Rhodium (Rh) | Osmium (Os) |
|---|---|---|
| Atomic Number | 45 | 76 |
| Density (g/cm3) | 12.41 | 22.59 |
| Melting Point (degC) | 1964 | 3033 |
| Catalytic Activity | High, especially in hydrogenation and automotive catalytic converters | Moderate, mainly used in specialized oxidation reactions |
| Corrosion Resistance | Excellent | Good but can oxidize forming volatile OsO4 |
| Cost | Very high, widely used in industry | High, limited industrial catalyst use |
| Common Applications as Catalyst | Automotive catalytic converters, chemical synthesis, hydrogenation | Specialized oxidation catalysts, scientific research |
Introduction to Rhodium and Osmium as Catalysts
Rhodium and osmium, members of the platinum group metals, serve as highly effective catalysts in various chemical reactions due to their unique electronic configurations and surface properties. Rhodium exhibits exceptional catalytic activity in hydrogenation and carbon monoxide oxidation processes, making it invaluable in automotive catalytic converters and fine chemical synthesis. Osmium, known for its robustness and high oxidation states, catalyzes reactions such as ammonia synthesis and oxidation reactions, though its rarity and toxicity limit widespread industrial application.
Chemical Properties Influencing Catalytic Activity
Rhodium exhibits exceptional catalytic activity due to its strong affinity for nitrogen compounds and its ability to facilitate hydrogenation reactions through effective adsorption and activation of reactants. Osmium, characterized by a higher density and unique electronic structure, offers distinct catalytic properties but is often less favored because of its tendency to form stable oxides that can inhibit surface reactions. The superior catalytic performance of rhodium in processes like automotive exhaust treatment and hydroformylation stems from its optimal balance of electron configuration and surface reactivity compared to osmium.
Abundance and Availability in Nature
Rhodium occurs at approximately 0.001 parts per million in the Earth's crust, making it one of the rarest platinum-group metals, while osmium is found at about 0.004 parts per million, slightly more abundant but still scarce. Both metals are primarily extracted from nickel and platinum ores, with rhodium often recovered as a byproduct of nickel mining in South Africa and Russia, and osmium mainly sourced from osmiridium found in alluvial deposits. The limited natural abundance and geographically concentrated deposits contribute to high market prices and impact their availability for catalytic applications.
Common Applications in Catalysis
Rhodium and osmium catalysts are extensively used in automotive catalytic converters, with rhodium primarily facilitating the reduction of nitrogen oxides (NOx) and osmium employed in specialized hydrogenation reactions due to its high catalytic activity. Rhodium's exceptional selectivity and resistance to poisoning make it ideal for processes like hydroformylation and selective catalytic reduction (SCR) in cleaning exhaust emissions. Osmium's stability under harsh conditions supports its use in ammonia synthesis and alkene hydrogenation, highlighting its role in industrial chemical manufacturing despite its rarity.
Efficiency and Selectivity in Catalytic Reactions
Rhodium exhibits superior catalytic efficiency in hydrogenation and hydroformylation reactions due to its exceptional ability to activate molecular hydrogen and carbon monoxide under mild conditions. Osmium, while less efficient, offers unique selectivity in specific oxidation reactions, such as dihydroxylation of alkenes, attributed to its high oxidation state stability. The choice between rhodium and osmium catalysts hinges on the desired balance between reaction rate and product selectivity in industrial chemical processes.
Cost Comparison and Economic Feasibility
Rhodium catalysts generally exhibit higher cost efficiency compared to osmium due to rhodium's greater availability and lower market price, which can reduce total production expenses. Osmium, while possessing excellent catalytic properties, is significantly rarer and more expensive, leading to limited economic feasibility in large-scale applications. Cost-effective industrial catalytic processes often favor rhodium to balance performance with budget constraints, optimizing overall economic viability.
Environmental Impact and Sustainability
Rhodium catalysts exhibit superior durability and high catalytic efficiency in automotive catalytic converters, significantly reducing nitrogen oxide emissions and contributing to cleaner air quality. Osmium, while highly effective in certain industrial oxidation reactions, poses environmental challenges due to its rarity and the toxic nature of osmium tetroxide, which raises sustainability concerns. The widespread use of rhodium is favored in green technologies for its balance of catalytic performance and lower ecological risks compared to osmium's limited application and higher toxicity.
Stability and Durability Under Reaction Conditions
Rhodium exhibits exceptional stability and durability under harsh reaction conditions, maintaining catalytic activity at high temperatures and in aggressive chemical environments. Osmium, while possessing excellent catalytic properties, tends to form volatile and toxic oxides, which can reduce its long-term stability and durability in catalytic processes. Rhodium's resistance to oxidation and structural degradation makes it a preferred choice for stable and durable catalysts in industrial applications.
Recent Advances and Research Trends
Rhodium and osmium, both platinum-group metals, show distinct catalytic properties with rhodium excelling in automotive catalytic converters due to its superior NOx reduction efficiency and osmium emerging in niche hydrogenation and electrochemical catalysis applications. Recent advances highlight rhodium nanoparticles engineered with tailored surface morphologies to enhance selectivity and durability in catalytic cycles, while osmium-based catalysts benefit from innovative alloying strategies that improve their stability and activity under harsh reaction conditions. Research trends emphasize sustainable catalyst design, leveraging atomic-level modifications and support materials to optimize performance and reduce precious metal loadings in both rhodium and osmium catalytic systems.
Choosing the Right Metal: Rhodium vs Osmium
Rhodium offers superior catalytic selectivity and resistance to poisoning, making it ideal for automotive catalytic converters and fine chemical synthesis. Osmium provides exceptional durability and high hardness but is less commonly used due to its toxicity and cost. When choosing between the two, rhodium is preferred for environmentally sensitive applications, while osmium suits specialized industrial processes requiring extreme wear resistance.
Infographic: Rhodium vs Osmium for Catalyst
 azmater.com
azmater.com