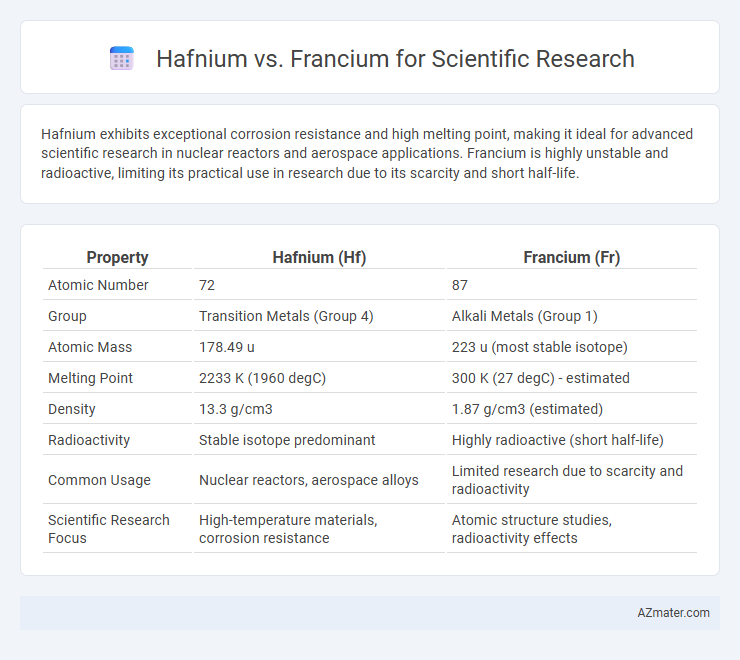
Hafnium exhibits exceptional corrosion resistance and high melting point, making it ideal for advanced scientific research in nuclear reactors and aerospace applications. Francium is highly unstable and radioactive, limiting its practical use in research due to its scarcity and short half-life.
Table of Comparison
| Property | Hafnium (Hf) | Francium (Fr) |
|---|---|---|
| Atomic Number | 72 | 87 |
| Group | Transition Metals (Group 4) | Alkali Metals (Group 1) |
| Atomic Mass | 178.49 u | 223 u (most stable isotope) |
| Melting Point | 2233 K (1960 degC) | 300 K (27 degC) - estimated |
| Density | 13.3 g/cm3 | 1.87 g/cm3 (estimated) |
| Radioactivity | Stable isotope predominant | Highly radioactive (short half-life) |
| Common Usage | Nuclear reactors, aerospace alloys | Limited research due to scarcity and radioactivity |
| Scientific Research Focus | High-temperature materials, corrosion resistance | Atomic structure studies, radioactivity effects |
Introduction to Hafnium and Francium
Hafnium (Hf), a transition metal with atomic number 72, is valued in scientific research for its high melting point, corrosion resistance, and applications in nuclear reactors and aerospace components. Francium (Fr), with atomic number 87, is a highly radioactive alkali metal, notable for its scarcity and instability, making it primarily useful in fundamental atomic structure studies and rare radioactive decay experiments. Research involving hafnium often centers on material science and nuclear technology, whereas francium's use is limited to specialized physics research due to its short half-life and extreme rarity.
Elemental Properties Comparison
Hafnium exhibits superior chemical stability and a high melting point of 2233degC, making it ideal for high-temperature and nuclear reactor applications, unlike Francium which is highly radioactive with a half-life of just 22 minutes, limiting its practical use. Hafnium's electron configuration [Xe] 4f14 5d2 6s2 allows efficient d-orbital involvement in bonding, whereas Francium, an alkali metal with electron configuration [Rn] 7s1, shows extreme reactivity and radioactivity. The marked differences in atomic number--Hafnium at 72 and Francium at 87--and their placement in the periodic table influence their stability and functionality in scientific experiments.
Abundance and Natural Occurrence
Hafnium, found predominantly in zirconium minerals, has an abundance of about 5.8 ppm in the Earth's crust, making it readily available for scientific research involving materials science and nuclear technology. Francium, with its extreme rarity and existence only as trace amounts produced by radioactive decay, poses significant challenges for research due to its scarcity and high radioactivity, limiting its practical applications. The natural occurrence and relative abundance of hafnium provide a more accessible element for experimental studies compared to francium's transient, highly unstable presence.
Isotopic Variations and Stability
Hafnium offers a diverse range of stable isotopes, with Hf-176 being the most abundant and useful for precise radiometric dating in geological research. Francium, on the other hand, has no stable isotopes and exists only in highly radioactive, short-lived forms such as Fr-223, limiting its practical applications in scientific studies. The isotopic stability of hafnium enhances its use in nuclear science and material analysis, whereas francium's extreme instability confines research primarily to theoretical and experimental physics.
Experimental Handling and Safety Considerations
Hafnium, a high-melting-point transition metal, offers stability and ease of experimental handling due to its low radioactivity and robust chemical resistance, making it suitable for precise scientific measurements and applications in nuclear reactors. Francium, an extremely rare and highly radioactive alkali metal with a half-life of only about 22 minutes, presents significant safety challenges, including intense radiation hazards and rapid decay, which severely limit its experimental use and require stringent containment protocols. Researchers prioritize hafnium for long-term experiments requiring material durability and safety, while francium's applications remain primarily theoretical or in brief, highly controlled experimental setups.
Applications in Scientific Research
Hafnium exhibits exceptional properties such as high melting point and neutron absorption, making it invaluable in nuclear reactors and aerospace research for radiation shielding and control rod applications. Francium's extreme radioactivity and scarcity limit its use primarily to fundamental physics experiments studying atomic structure and decay, contributing to nuclear science and element behavior analysis. Researchers prioritize hafnium for practical applications requiring stability and durability, whereas francium provides insights into atomic interactions and nuclear synthesis processes.
Role in Nuclear Science and Technology
Hafnium plays a crucial role in nuclear science due to its high neutron absorption cross-section, making it essential for control rods in nuclear reactors to manage fission reactions safely. Francium, being highly radioactive and extremely rare, has limited practical applications but is valuable in fundamental scientific research to study atomic structure and nuclear decay processes. The contrasting properties of hafnium's stability and francium's radioactivity highlight their distinct contributions to nuclear technology and experimental nuclear physics.
Analytical Methods for Detection
Hafnium's stable isotopes enable precise detection through inductively coupled plasma mass spectrometry (ICP-MS) and X-ray fluorescence (XRF), facilitating accurate quantification in materials science and geochemistry. Francium, characterized by extreme radioactivity and scarcity, requires specialized radiometric techniques like alpha spectroscopy and gamma-ray spectrometry for detection, primarily in nuclear physics research. Analytical challenges in francium detection arise from its short half-life and low natural abundance, demanding sensitive instrumentation and rapid measurement protocols.
Challenges in Acquisition and Cost
Hafnium is more accessible for scientific research due to its relative abundance in zirconium ores and established extraction methods, whereas francium's extreme rarity and high radioactivity create significant challenges in acquisition. The cost of hafnium remains moderate, supported by existing industrial demand, while francium's production relies on particle accelerators, making it prohibitively expensive and available only in trace amounts. These factors limit francium use primarily to theoretical studies and specialized nuclear experiments, whereas hafnium finds broader application in materials science and nuclear reactors.
Future Prospects in Research
Hafnium's stable isotopes and high melting point make it a promising candidate for advanced nuclear reactors and materials science research, driving innovations in energy and aerospace technologies. Francium's extreme radioactivity and scarcity limit its experimental use, but potential applications in atomic structure studies and fundamental physics could yield breakthroughs in quantum mechanics and nuclear decay processes. Future research prospects favor hafnium for practical technological advancements, while francium remains pivotal for theoretical exploration and understanding of exotic atomic behaviors.
Infographic: Hafnium vs Francium for Scientific Research
 azmater.com
azmater.com