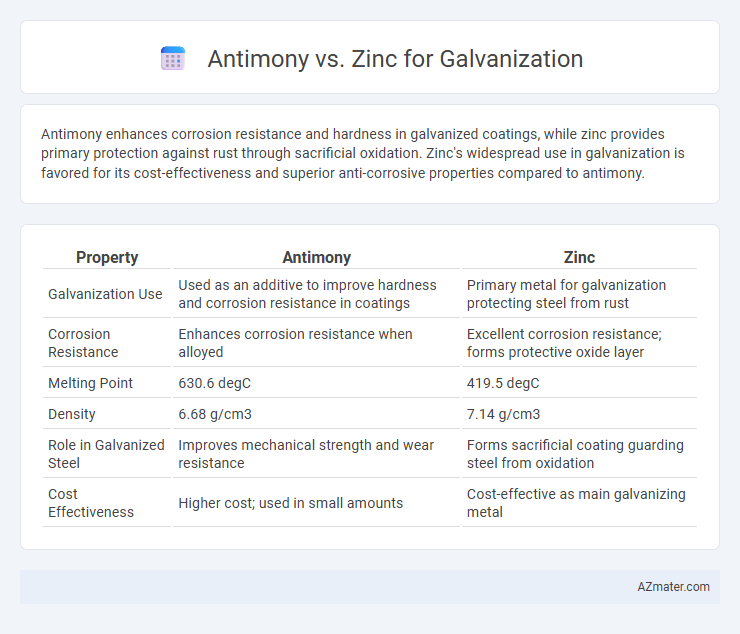
Antimony enhances corrosion resistance and hardness in galvanized coatings, while zinc provides primary protection against rust through sacrificial oxidation. Zinc's widespread use in galvanization is favored for its cost-effectiveness and superior anti-corrosive properties compared to antimony.
Table of Comparison
| Property | Antimony | Zinc |
|---|---|---|
| Galvanization Use | Used as an additive to improve hardness and corrosion resistance in coatings | Primary metal for galvanization protecting steel from rust |
| Corrosion Resistance | Enhances corrosion resistance when alloyed | Excellent corrosion resistance; forms protective oxide layer |
| Melting Point | 630.6 degC | 419.5 degC |
| Density | 6.68 g/cm3 | 7.14 g/cm3 |
| Role in Galvanized Steel | Improves mechanical strength and wear resistance | Forms sacrificial coating guarding steel from oxidation |
| Cost Effectiveness | Higher cost; used in small amounts | Cost-effective as main galvanizing metal |
Introduction to Galvanization
Galvanization involves coating metals such as steel or iron with zinc to prevent corrosion and extend their lifespan. Zinc is the primary material used due to its sacrificial oxidation properties, which protect the underlying metal. The addition of antimony in zinc alloys can enhance hardness and improve corrosion resistance, optimizing the overall protective quality of the galvanized coating.
Overview of Antimony in Galvanization
Antimony enhances the corrosion resistance and hardness of galvanized coatings by refining the microstructure of zinc layers. It acts as a grain refiner, improving coating adhesion and durability in harsh environments. The addition of antimony in zinc galvanization increases the lifespan of the protective layer, making it ideal for industrial and marine applications.
Overview of Zinc in Galvanization
Zinc is the primary metal used in galvanization due to its excellent corrosion resistance and ability to form a protective oxide layer on steel surfaces. Unlike antimony, which is rarely used in galvanization, zinc offers a durable and cost-effective solution to prevent rusting in steel structures. Its widespread application in industries such as construction and automotive demonstrates zinc's superior performance in enhancing metal longevity.
Chemical Properties: Antimony vs Zinc
Antimony exhibits a higher melting point (630.6degC) and greater chemical stability compared to zinc, which melts at 419.5degC and oxidizes more readily in air. Zinc's amphoteric nature allows it to react with both acids and bases, enhancing its protective galvanization capabilities, while antimony is less reactive and mainly used as an alloying agent to improve hardness. The differing oxidation states--antimony commonly in +3 and +5, versus zinc's stable +2--significantly influence their corrosion resistance and effectiveness in galvanization processes.
Corrosion Resistance Comparison
Antimony enhances zinc coatings in galvanization by improving corrosion resistance through increased hardness and reduced porosity, which slows the penetration of corrosive agents. Zinc alone provides effective sacrificial protection by forming a stable oxide layer, but the addition of antimony refines the microstructure, resulting in a more durable and longer-lasting protective barrier. Studies indicate that zinc-antimony alloys exhibit superior performance in harsh environments compared to pure zinc coatings, making them preferable for applications requiring enhanced corrosion protection.
Cost Analysis: Antimony vs Zinc
Antimony is significantly more expensive than zinc, with market prices often exceeding zinc by several times due to its scarcity and specialized applications. Zinc's lower cost and abundant availability make it the preferred choice for large-scale galvanization projects, offering cost-effective corrosion protection. The higher price of antimony limits its use to niche applications where its unique properties justify the increased expense.
Environmental Impact and Sustainability
Antimony and zinc differ significantly in their environmental impact and sustainability for galvanization applications. Zinc, widely used in galvanization, offers greater recyclability and lower toxicity, contributing to reduced heavy metal contamination in ecosystems. Conversely, antimony mining generates substantial hazardous waste and poses higher ecological risks, making zinc a more sustainable choice for corrosion protection.
Applications and Industrial Usages
Antimony enhances the corrosion resistance and mechanical strength of zinc coatings, making it ideal for heavy-duty galvanization in construction and automotive industries where durability is critical. Zinc remains the primary metal for galvanization due to its excellent sacrificial protection properties, commonly used in infrastructure, electrical components, and household appliances. Industrial applications favor antimony-alloyed zinc coatings for increased hardness and wear resistance, while pure zinc galvanization is preferred for cost-effective, large-scale protection against rust.
Durability and Performance Differences
Antimony enhances zinc coatings' hardness and corrosion resistance, making galvanized steel more durable in harsh environments. Zinc alone provides excellent sacrificial protection but is softer and less resistant to mechanical wear compared to zinc-antimony alloys. The addition of antimony improves performance by reducing coating degradation and extending the lifespan of galvanized materials under industrial exposure.
Conclusion: Choosing the Right Metal for Galvanization
Antimony enhances zinc coatings by improving hardness and corrosion resistance, making it ideal for heavy-duty applications requiring durability. Zinc alone provides excellent corrosion protection with cost-effectiveness and ease of application, suitable for general galvanization tasks. Selecting the right metal depends on the specific environmental exposure and mechanical demands, with zinc-antimony alloys favored for superior performance in aggressive conditions.
Infographic: Antimony vs Zinc for Galvanization
 azmater.com
azmater.com