Gold offers superior corrosion resistance and excellent electrical conductivity, making it ideal for high-reliability electronic components. Iron provides cost-effective structural support but lacks the conductivity and oxidation resistance essential for sensitive electronic applications.
Table of Comparison
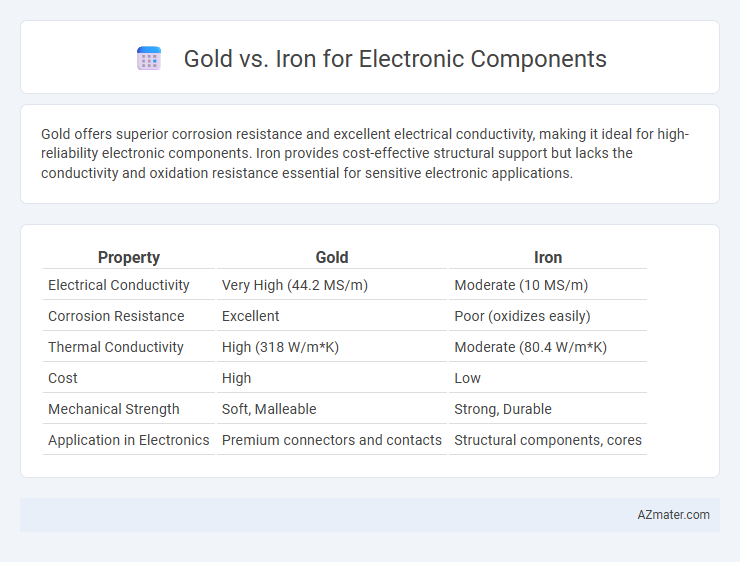
| Property | Gold | Iron |
|---|---|---|
| Electrical Conductivity | Very High (44.2 MS/m) | Moderate (10 MS/m) |
| Corrosion Resistance | Excellent | Poor (oxidizes easily) |
| Thermal Conductivity | High (318 W/m*K) | Moderate (80.4 W/m*K) |
| Cost | High | Low |
| Mechanical Strength | Soft, Malleable | Strong, Durable |
| Application in Electronics | Premium connectors and contacts | Structural components, cores |
Introduction: Gold vs Iron in Electronic Components
Gold offers superior corrosion resistance and excellent electrical conductivity, making it ideal for high-reliability electronic components such as connectors and circuit boards. Iron, while more cost-effective and mechanically robust, is prone to oxidation and less conductive, limiting its use primarily to structural or magnetic components within electronics. The distinct material properties of gold and iron influence their application based on performance requirements and budget constraints in electronic manufacturing.
Material Properties and Conductivity
Gold exhibits superior corrosion resistance and excellent electrical conductivity, making it ideal for electronic components exposed to harsh environments. Iron, while more abundant and cost-effective, has lower conductivity and is prone to oxidation, which can degrade performance. The choice between gold and iron hinges on balancing conductivity, durability, and material cost for specific electronic applications.
Corrosion Resistance and Longevity
Gold offers superior corrosion resistance compared to iron, making it an ideal choice for electronic components exposed to harsh environments. Its inert nature prevents oxidation and tarnishing, ensuring stable conductivity and long-term reliability in circuits. Iron, while cost-effective, is prone to rust and corrosion, which can degrade performance and reduce the lifespan of electronic parts.
Electrical Performance Comparison
Gold outperforms iron in electronic components due to its superior electrical conductivity and resistance to corrosion, ensuring stable signal transmission and longevity. Iron, with lower conductivity and susceptibility to oxidation, can cause higher resistance and signal degradation over time. Gold's inertness and reliable contact performance make it the preferred choice for high-frequency and precision applications where minimal electrical loss is critical.
Cost Analysis: Gold vs Iron
Gold offers superior corrosion resistance and excellent conductivity, making it ideal for high-performance electronic components despite its significantly higher cost compared to iron. Iron, although much cheaper and more abundant, suffers from oxidation and lower electrical conductivity, which can increase maintenance costs and reduce component lifespan. Cost analysis often favors iron for budget-sensitive projects, but gold's reliability and efficiency justify its premium in critical applications such as connectors and semiconductor packaging.
Applications in Modern Electronics
Gold offers superior corrosion resistance and excellent electrical conductivity, making it ideal for reliable connectors, switches, and semiconductor contacts in modern electronics. Iron, while cost-effective and magnetic, is primarily used in inductors, transformers, and electromagnetic components where its magnetic properties are crucial. The preference for gold in high-performance and precision applications ensures long-term durability and minimal signal loss compared to iron-based parts.
Influence on Signal Integrity
Gold's excellent conductivity and resistance to oxidation ensure stable signal transmission in electronic components, minimizing signal loss and noise interference. In contrast, iron's higher resistivity and susceptibility to corrosion often cause signal degradation and increased electromagnetic interference. Therefore, gold-plated contacts are preferred in high-frequency circuits to maintain optimal signal integrity and reliability.
Manufacturability and Scalability
Gold offers superior corrosion resistance and excellent electrical conductivity, making it highly reliable for electronic components, but its high cost and limited availability challenge large-scale manufacturing. Iron provides a cost-effective alternative with adequate magnetic properties and good mechanical strength, enhancing scalability in mass production despite lower conductivity and susceptibility to oxidation. Manufacturers often balance the trade-offs by using gold for critical connectors and iron-based alloys for structural parts to optimize both performance and production efficiency.
Environmental and Sustainability Considerations
Gold is highly valued in electronic components for its excellent corrosion resistance and superior electrical conductivity, which enhance device longevity and reduce electronic waste. Iron, while more abundant and less costly, tends to oxidize and degrade faster, leading to increased maintenance and replacement rates that negatively impact sustainability. Choosing gold over iron supports longer-lasting electronics and lowers the environmental footprint by minimizing resource consumption and e-waste generation.
Future Trends in Electronic Material Selection
Gold remains a prime choice in electronic components for its excellent corrosion resistance and superior conductivity, crucial for high-reliability applications in aerospace and medical devices. Iron, while less conductive, is increasingly integrated into magnetic components and emerging spintronic devices due to its abundant availability and magnetic properties. Future trends indicate a growing emphasis on hybrid materials combining gold's stability with iron-based compounds to enhance performance and reduce costs in next-generation electronics.
Infographic: Gold vs Iron for Electronic Component
 azmater.com
azmater.com