Amorphous metals offer superior electrical conductivity, corrosion resistance, and flexibility compared to gold in electronics applications. Their unique non-crystalline structure enhances durability and reduces manufacturing costs, making them a promising alternative to traditional gold components.
Table of Comparison
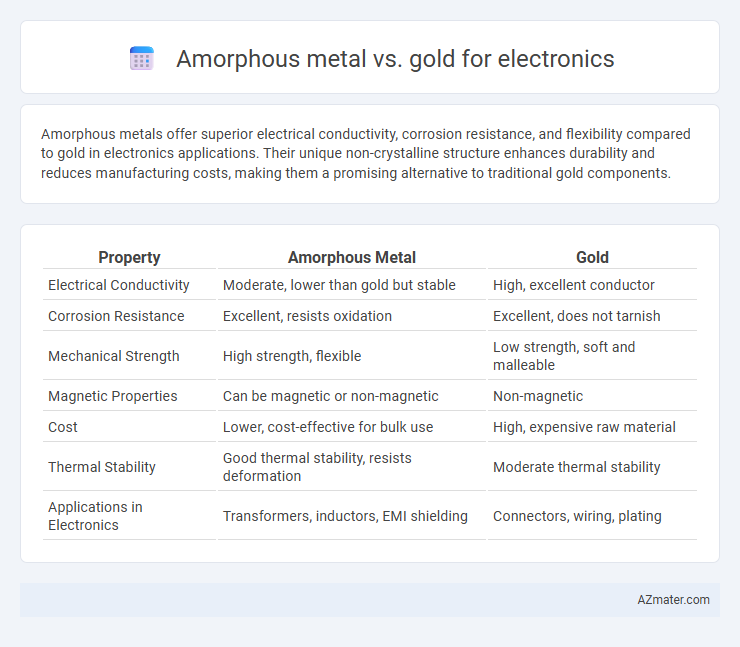
| Property | Amorphous Metal | Gold |
|---|---|---|
| Electrical Conductivity | Moderate, lower than gold but stable | High, excellent conductor |
| Corrosion Resistance | Excellent, resists oxidation | Excellent, does not tarnish |
| Mechanical Strength | High strength, flexible | Low strength, soft and malleable |
| Magnetic Properties | Can be magnetic or non-magnetic | Non-magnetic |
| Cost | Lower, cost-effective for bulk use | High, expensive raw material |
| Thermal Stability | Good thermal stability, resists deformation | Moderate thermal stability |
| Applications in Electronics | Transformers, inductors, EMI shielding | Connectors, wiring, plating |
Introduction to Amorphous Metals and Gold in Electronics
Amorphous metals, also known as metallic glasses, exhibit a non-crystalline atomic structure that provides superior strength, corrosion resistance, and soft magnetic properties beneficial in electronic components like transformers and sensors. Gold is prized in electronics for its excellent electrical conductivity, resistance to oxidation, and reliable performance in connectors, switches, and bonding wires. While gold maintains electrical integrity under harsh conditions, amorphous metals contribute to miniaturization and efficiency improvements in advanced electronic devices due to their unique mechanical and magnetic characteristics.
Fundamental Properties of Amorphous Metals
Amorphous metals, also known as metallic glasses, exhibit a disordered atomic structure that provides unique magnetic, electrical, and mechanical properties distinct from crystalline gold. Their high strength, corrosion resistance, and excellent soft magnetic characteristics make them ideal for efficient electronic components, surpassing gold's traditional role in conductivity. Unlike gold, amorphous metals offer reduced eddy current losses and enhanced miniaturization potential in electronic devices due to their low electrical resistivity and superior thermal stability.
Gold: Traditional Choice for Electronic Components
Gold remains the traditional choice for electronic components due to its excellent electrical conductivity, resistance to corrosion, and superior reliability in extreme environments. Unlike amorphous metals, which offer unique magnetic properties and mechanical strength, gold ensures optimal signal integrity and consistent performance in connectors, switches, and circuit boards. Its stability and durability in microelectronics make gold indispensable despite higher costs compared to emerging materials.
Electrical Conductivity: Amorphous Metal vs Gold
Amorphous metals exhibit lower electrical conductivity compared to gold, which is renowned for its exceptional conductivity of approximately 45.2 x 10^6 S/m at room temperature. The disordered atomic structure of amorphous metals disrupts electron flow, resulting in higher electrical resistivity. Gold's superior conductivity and resistance to oxidation make it the preferred material for high-performance electronic connections and components.
Thermal Stability and Performance Comparison
Amorphous metals exhibit superior thermal stability compared to gold, maintaining structural integrity at higher temperatures due to their non-crystalline atomic arrangement, which prevents grain boundary diffusion common in crystalline metals like gold. In electronic applications, this enhanced thermal stability translates to improved performance and longevity under thermal stress, reducing failure rates in high-temperature environments. While gold offers excellent electrical conductivity, amorphous metals provide a compelling balance of electrical and thermal properties, making them advantageous for advanced electronic components requiring robust thermal management.
Corrosion and Wear Resistance in Electronic Applications
Amorphous metals exhibit superior corrosion resistance compared to gold in electronic applications, due to their non-crystalline structure that prevents grain boundary corrosion and enhances chemical stability. Gold is inherently corrosion-resistant but is prone to wear and mechanical degradation under repeated stress or abrasive contact. The superior wear resistance of amorphous metals makes them more suitable for durable electronic components exposed to harsh environments, reducing maintenance and extending device lifespan.
Manufacturing and Cost Implications
Amorphous metals offer superior electrical resistivity and magnetic properties compared to gold, making them advantageous for advanced electronic components with reduced energy losses. Manufacturing amorphous metal components typically involves rapid cooling processes like melt spinning, which can be more cost-effective and scalable than traditional gold-based fabrication requiring precious metal procurement and complex plating techniques. Cost implications favor amorphous metals as they reduce reliance on expensive raw materials like gold while enabling efficient mass production and improved device performance in electronic applications.
Applications in Modern Electronic Devices
Amorphous metal offers exceptional soft magnetic properties and high corrosion resistance, making it ideal for inductors, transformers, and magnetic sensors in modern electronics. Gold is extensively used in electronic connectors, bonding wires, and printed circuit boards due to its excellent electrical conductivity and resistance to oxidation. While amorphous metals improve energy efficiency and miniaturization in power electronics, gold ensures reliable signal transmission and durability in high-performance devices.
Environmental Impact and Sustainability
Amorphous metals offer significant environmental benefits over gold in electronics due to their abundant raw materials and lower energy consumption in manufacturing processes. Gold extraction is resource-intensive, involving toxic chemicals like cyanide, which causes substantial ecological damage and pollution. The recyclability of amorphous metals enhances sustainability by reducing electronic waste and minimizing reliance on limited gold reserves.
Future Prospects: Amorphous Metals Replacing Gold?
Amorphous metals exhibit superior corrosion resistance, enhanced mechanical strength, and cost-effectiveness compared to gold, making them promising candidates for future electronic applications. Innovations in amorphous metal alloys and deposition techniques enable improved electrical conductivity and reliability in microelectronics. These advancements indicate a potential shift where amorphous metals could increasingly replace gold in components such as connectors, bonding wires, and conductive coatings, optimizing performance and reducing costs.
Infographic: Amorphous metal vs Gold for Electronics
 azmater.com
azmater.com