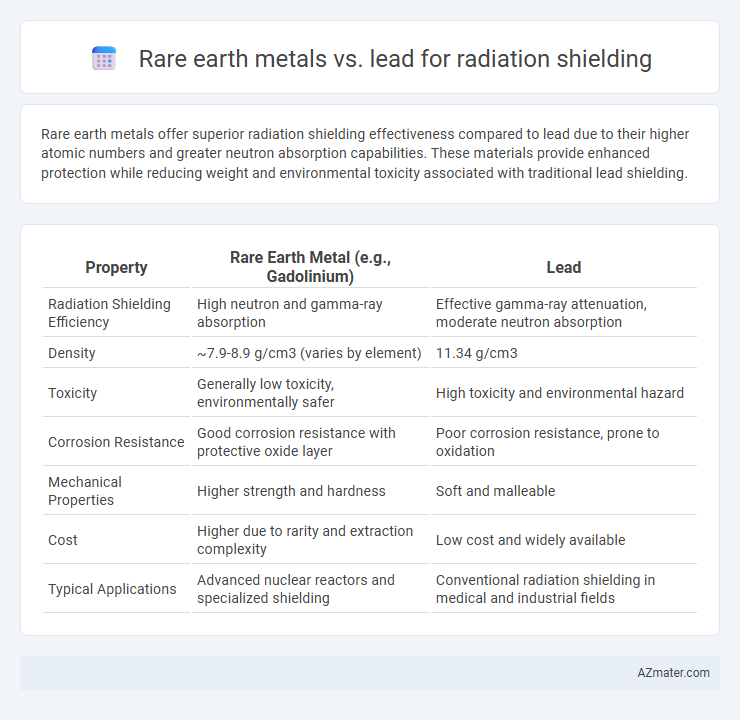
Rare earth metals offer superior radiation shielding effectiveness compared to lead due to their higher atomic numbers and greater neutron absorption capabilities. These materials provide enhanced protection while reducing weight and environmental toxicity associated with traditional lead shielding.
Table of Comparison
| Property | Rare Earth Metal (e.g., Gadolinium) | Lead |
|---|---|---|
| Radiation Shielding Efficiency | High neutron and gamma-ray absorption | Effective gamma-ray attenuation, moderate neutron absorption |
| Density | ~7.9-8.9 g/cm3 (varies by element) | 11.34 g/cm3 |
| Toxicity | Generally low toxicity, environmentally safer | High toxicity and environmental hazard |
| Corrosion Resistance | Good corrosion resistance with protective oxide layer | Poor corrosion resistance, prone to oxidation |
| Mechanical Properties | Higher strength and hardness | Soft and malleable |
| Cost | Higher due to rarity and extraction complexity | Low cost and widely available |
| Typical Applications | Advanced nuclear reactors and specialized shielding | Conventional radiation shielding in medical and industrial fields |
Introduction to Radiation Shielding Materials
Rare earth metals such as gadolinium and dysprosium offer high neutron absorption cross-sections, making them effective for radiation shielding compared to traditional materials like lead. Lead is widely used due to its high density and ability to attenuate gamma rays, but rare earth metals provide enhanced performance in neutron radiation environments. Combining rare earth metals with lead can optimize shielding effectiveness across diverse radiation types, balancing weight and protection.
Understanding Rare Earth Metals
Rare earth metals such as cerium, gadolinium, and lanthanum exhibit high neutron absorption cross-sections, making them effective for radiation shielding compared to lead. These metals provide superior attenuation of gamma and neutron radiation due to their unique electronic structures and dense atomic arrangements. Understanding the intrinsic properties of rare earth metals allows for the development of lightweight, efficient shielding materials that outperform traditional lead-based solutions in nuclear and medical applications.
Properties of Lead as a Shielding Material
Lead demonstrates exceptional radiation shielding capabilities due to its high density (11.34 g/cm3) and atomic number (82), which effectively attenuate gamma rays and x-rays. Its malleability and corrosion resistance enable easy fabrication into shields of various shapes and thicknesses, making it a versatile material for protective barriers in medical, industrial, and nuclear applications. The toxicity of lead, however, necessitates careful handling and containment during its manufacture and use in radiation shielding.
Radiation Attenuation Capabilities: Rare Earth Metals vs. Lead
Rare earth metals exhibit superior radiation attenuation capabilities compared to lead due to their higher atomic numbers and densities, which enhance their effectiveness in absorbing gamma rays and X-rays. Materials like gadolinium and dysprosium provide increased neutron capture cross-sections, making them more efficient for neutron radiation shielding than traditional lead shields. While lead remains widely used for its cost-effectiveness and ease of fabrication, rare earth metals offer enhanced protection in high-performance radiation shielding applications where weight and attenuation efficiency are critical factors.
Density and Thickness Requirements for Effective Shielding
Rare earth metals exhibit higher densities than lead, with elements like Gadolinium reaching densities around 7.9 g/cm3, making them competitive for radiation shielding applications requiring compact barriers. Effective shielding depends on both density and thickness; rare earth metals can achieve comparable attenuation to lead at reduced thicknesses due to their high atomic numbers and neutron absorption cross-sections. However, the optimal use of rare earth metals often involves balancing material costs and mechanical properties while meeting stringent thickness requirements for gamma and neutron radiation attenuation.
Toxicity and Environmental Impact Comparison
Rare earth metals offer effective radiation shielding with significantly lower toxicity compared to lead, which is highly toxic and poses severe environmental hazards during extraction, use, and disposal. Lead contamination can cause long-term soil and water pollution, biomagnification, and adverse health effects on humans and wildlife, while rare earth metals, though requiring careful mining practices, generally present fewer environmental risks. The shift towards rare earth metals in shielding applications supports safer handling and reduced ecological footprint in medical, industrial, and nuclear sectors.
Mechanical and Structural Performance
Rare earth metals such as tungsten and gadolinium exhibit superior mechanical strength and higher density compared to lead, resulting in enhanced radiation shielding efficiency while maintaining structural integrity under stress. These metals provide better resistance to deformation and wear, making them ideal for applications requiring durable, long-lasting shields in harsh environments. Their high melting points and corrosion resistance further contribute to improved performance in demanding mechanical and structural roles.
Cost and Economic Considerations
Rare earth metals, despite offering superior radiation shielding efficiency due to their high atomic numbers and density, typically incur higher costs than lead, making them less economically viable for large-scale applications. Lead remains the preferred material in radiation shielding because of its relatively low price, availability, and ease of manufacturing, which significantly reduces overall project expenses. However, rare earth metals may present cost benefits in specialized settings where enhanced performance justifies the initial investment and long-term durability reduces replacement frequency.
Emerging Applications and Future Trends
Rare earth metals, such as gadolinium and dysprosium, exhibit superior neutron absorption capabilities compared to lead, making them increasingly valuable in advanced radiation shielding applications for nuclear medicine and space exploration. Emerging technologies leverage these metals' high density and magnetic properties to develop lightweight, more effective shields that address the limitations of traditional lead-based materials in weight and toxicity. Future trends indicate a growing adoption of rare earth composites and nanostructured materials to enhance radiation protection performance while reducing environmental impact.
Conclusion: Choosing the Optimal Radiation Shield
Rare earth metals offer superior radiation shielding efficiency compared to lead due to their higher atomic numbers and greater density, enabling better attenuation of gamma and X-rays. Despite lead's cost-effectiveness and widespread use, rare earth metals provide enhanced protection in critical applications such as medical imaging and nuclear reactors. Selecting the optimal radiation shield depends on balancing shielding performance, cost, weight, and environmental considerations, with rare earth metals emerging as a promising alternative for advanced, high-performance radiation protection.
Infographic: Rare earth metal vs Lead for Radiation Shielding
 azmater.com
azmater.com