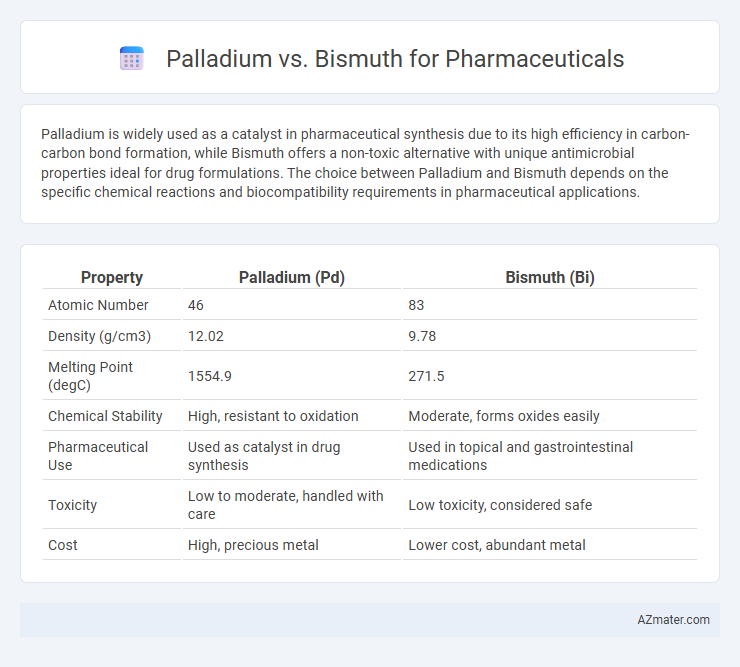
Palladium is widely used as a catalyst in pharmaceutical synthesis due to its high efficiency in carbon-carbon bond formation, while Bismuth offers a non-toxic alternative with unique antimicrobial properties ideal for drug formulations. The choice between Palladium and Bismuth depends on the specific chemical reactions and biocompatibility requirements in pharmaceutical applications.
Table of Comparison
| Property | Palladium (Pd) | Bismuth (Bi) |
|---|---|---|
| Atomic Number | 46 | 83 |
| Density (g/cm3) | 12.02 | 9.78 |
| Melting Point (degC) | 1554.9 | 271.5 |
| Chemical Stability | High, resistant to oxidation | Moderate, forms oxides easily |
| Pharmaceutical Use | Used as catalyst in drug synthesis | Used in topical and gastrointestinal medications |
| Toxicity | Low to moderate, handled with care | Low toxicity, considered safe |
| Cost | High, precious metal | Lower cost, abundant metal |
Introduction to Palladium and Bismuth in Pharmaceuticals
Palladium and bismuth play distinct yet pivotal roles in pharmaceutical development, with palladium widely used as a catalyst in drug synthesis due to its efficiency in facilitating carbon-carbon bond formation and other key chemical reactions. Bismuth, valued for its low toxicity and antimicrobial properties, is commonly utilized in treatments for gastrointestinal disorders, including peptic ulcers and Helicobacter pylori infections. The unique chemical characteristics of palladium support innovative drug manufacturing processes, while bismuth's therapeutic applications contribute directly to patient care through effective and safe treatment options.
Chemical Properties: Palladium vs Bismuth
Palladium exhibits superior catalytic properties due to its ability to facilitate hydrogenation and carbon-carbon bond formation, making it valuable in pharmaceutical synthesis. Bismuth, characterized by low toxicity and unique Lewis acidity, serves as an eco-friendly catalyst in medicinal chemistry, particularly in drug formulation and antimicrobial applications. The distinct electronic configurations and coordination behaviors of palladium and bismuth dictate their roles in catalytic cycles and impact pharmaceutical reaction efficiencies.
Mechanisms of Action in Drug Synthesis
Palladium catalyzes carbon-carbon bond formation through mechanisms such as oxidative addition and reductive elimination, making it essential for cross-coupling reactions in drug synthesis. Bismuth operates via Lewis acid catalysis, facilitating selective activation of functional groups with lower toxicity and environmental impact. These distinct mechanisms influence their applications in synthesizing complex pharmaceutical compounds, with palladium favored for precision and bismuth for sustainable catalysis.
Safety and Toxicity Profiles
Palladium exhibits moderate toxicity with potential risks including allergic reactions and organ damage, necessitating careful handling in pharmaceutical applications, whereas bismuth is recognized for its low toxicity and established safety profile, often used safely in gastrointestinal treatments. Palladium compounds can cause environmental and biological accumulation concerns, while bismuth's minimal systemic absorption reduces long-term toxicity risks. Selecting bismuth over palladium in drug formulations prioritizes patient safety with fewer adverse effects and reduced toxicity liabilities.
Pharmaceutical Applications and Use Cases
Palladium plays a critical role in pharmaceutical applications as a catalyst for carbon-carbon coupling reactions, such as Suzuki and Heck couplings, enabling the efficient synthesis of complex drug molecules. Bismuth, renowned for its low toxicity and antimicrobial properties, is commonly used in gastroprotective formulations and as an active ingredient in treatments for Helicobacter pylori infections. The unique catalytic efficiency of palladium contrasts with bismuth's therapeutic applications, highlighting their complementary roles in drug development and safe medicinal use.
Catalytic Efficiency in Medicinal Chemistry
Palladium exhibits superior catalytic efficiency in medicinal chemistry due to its ability to facilitate cross-coupling reactions, enabling the synthesis of complex pharmaceutical compounds with high selectivity and yield. Bismuth, while less reactive, offers a non-toxic and environmentally benign alternative, making it valuable in green chemistry applications despite lower catalytic activity. The choice between palladium and bismuth catalysts hinges on balancing catalytic performance with safety and sustainability in drug development processes.
Environmental Impact and Sustainability
Palladium is widely used in pharmaceutical catalysis due to its high efficiency but poses environmental concerns due to toxicity and limited availability, raising sustainability issues. Bismuth offers a greener alternative with low toxicity, abundance, and biodegradability, reducing pharmaceutical manufacturing waste and environmental pollution. Transitioning to bismuth-based catalysts supports sustainable development goals by minimizing ecological footprint and preserving critical raw materials.
Cost Comparison: Palladium vs Bismuth
Palladium, widely used as a catalyst in pharmaceutical synthesis, typically incurs higher costs due to its rarity and demand in various industrial applications. Bismuth offers a more cost-effective alternative with lower market prices and sufficient catalytic properties for certain drug manufacturing processes. Choosing bismuth over palladium can significantly reduce production expenses while maintaining efficiency in pharmaceutical formulations.
Regulatory Considerations and Compliance
Palladium catalysts are widely used in pharmaceutical manufacturing due to their established regulatory acceptance and compliance with ICH guidelines, particularly concerning metal impurities limits defined by ICH Q3D. Bismuth, as a less common alternative, faces stricter scrutiny from regulatory bodies like the FDA and EMA due to limited toxicological and environmental data, demanding rigorous validation and risk assessment. Ensuring compliance requires comprehensive documentation of catalyst residue levels and demonstration of safe elimination or acceptable residual concentrations in the final drug product.
Future Trends in Pharmaceutical Metal Usage
Palladium remains a critical catalyst in pharmaceutical synthesis due to its efficiency in cross-coupling reactions and its established role in producing active pharmaceutical ingredients (APIs). Bismuth is gaining attention for its low toxicity and environmental benefits, making it a promising alternative in green chemistry applications within drug development. Future trends indicate increasing integration of bismuth in pharmaceutical formulations, driven by regulatory pressures to reduce heavy metal contamination and the demand for sustainable manufacturing processes.
Infographic: Palladium vs Bismuth for Pharmaceutical
 azmater.com
azmater.com