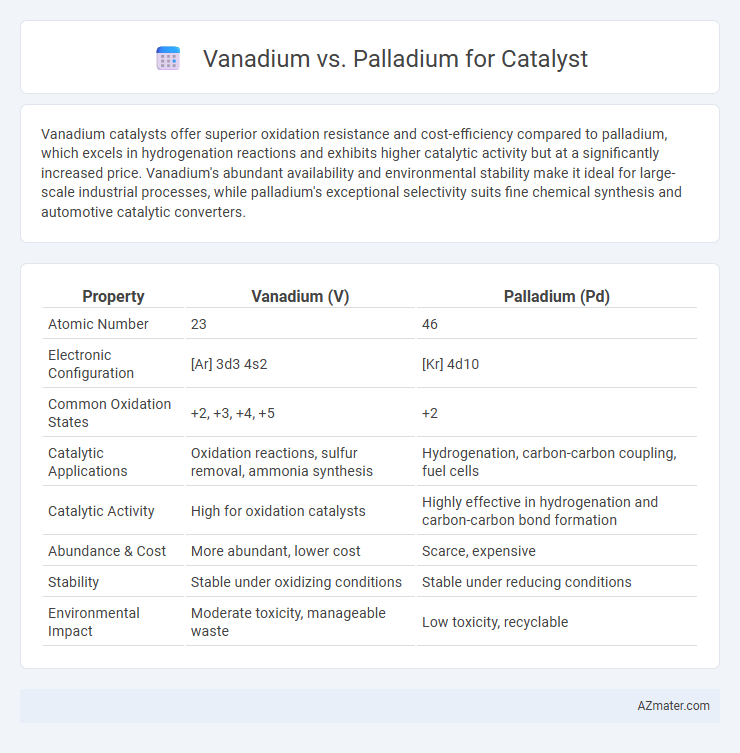
Vanadium catalysts offer superior oxidation resistance and cost-efficiency compared to palladium, which excels in hydrogenation reactions and exhibits higher catalytic activity but at a significantly increased price. Vanadium's abundant availability and environmental stability make it ideal for large-scale industrial processes, while palladium's exceptional selectivity suits fine chemical synthesis and automotive catalytic converters.
Table of Comparison
| Property | Vanadium (V) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 23 | 46 |
| Electronic Configuration | [Ar] 3d3 4s2 | [Kr] 4d10 |
| Common Oxidation States | +2, +3, +4, +5 | +2 |
| Catalytic Applications | Oxidation reactions, sulfur removal, ammonia synthesis | Hydrogenation, carbon-carbon coupling, fuel cells |
| Catalytic Activity | High for oxidation catalysts | Highly effective in hydrogenation and carbon-carbon bond formation |
| Abundance & Cost | More abundant, lower cost | Scarce, expensive |
| Stability | Stable under oxidizing conditions | Stable under reducing conditions |
| Environmental Impact | Moderate toxicity, manageable waste | Low toxicity, recyclable |
Introduction to Vanadium and Palladium as Catalysts
Vanadium and palladium are widely used transition metals in catalysis, each exhibiting unique properties that optimize their application in chemical reactions. Vanadium catalysts are particularly effective in oxidation processes and benefit from their variable oxidation states, enhancing redox reactions in industrial applications such as sulfuric acid production and selective oxidation of hydrocarbons. Palladium catalysts excel in hydrogenation, carbon-carbon coupling reactions like Suzuki and Heck reactions, due to their exceptional ability to activate molecular hydrogen and facilitate bond formation under mild conditions.
Chemical Properties: Vanadium vs Palladium
Vanadium exhibits multiple oxidation states ranging from +2 to +5, making it highly versatile for redox reactions in catalytic processes, particularly in sulfuric acid production and selective oxidation. Palladium, known for its stable +2 and +4 oxidation states, excels in hydrogenation and carbon-carbon coupling reactions due to its exceptional ability to adsorb hydrogen and activate organic molecules. The differing electronic configurations and coordination chemistry of vanadium and palladium govern their distinct catalytic behaviors, with vanadium favoring oxidative catalysis and palladium dominating hydrogenation and cross-coupling catalysis.
Catalytic Mechanisms: Key Differences
Vanadium catalysts operate primarily through redox mechanisms, cycling between multiple oxidation states to facilitate oxidation reactions, especially in sulfuric acid production and selective oxidation of hydrocarbons. Palladium catalysts typically function via adsorption and oxidative addition processes, enabling hydrogenation, carbon-carbon coupling, and oxidation reactions in fine chemical synthesis. The key difference lies in vanadium's ability to transfer oxygen atoms directly, whereas palladium excels in activating molecular hydrogen and promoting bond formation through surface interactions.
Applications in Industrial Catalysis
Vanadium catalysts excel in selective oxidation processes and sulfuric acid production due to their high thermal stability and oxygen transfer capabilities. Palladium catalysts dominate hydrogenation and carbon-carbon coupling reactions, essential in refining, pharmaceuticals, and automotive catalytic converters. The choice between vanadium and palladium depends on reaction type, feedstock, and desired selectivity, with vanadium favored for oxidation and palladium preferred for hydrogenation and coupling reactions.
Efficiency and Selectivity Comparison
Vanadium catalysts exhibit high oxidation efficiency in selective catalytic processes, particularly in sulfuric acid production and olefin polymerization, owing to their strong redox properties and ability to operate at elevated temperatures. Palladium catalysts offer superior selectivity and activity in hydrogenation and carbon-carbon coupling reactions, with exceptional tolerance to functional groups and lower overpotentials. Efficiency-wise, palladium generally provides faster reaction rates and higher product yields in fine chemical synthesis, whereas vanadium excels in large-scale industrial oxidation processes due to its robustness and cost-effectiveness.
Environmental Impact and Sustainability
Vanadium catalysts offer a lower environmental footprint due to their abundance and recyclability, contributing to reduced resource depletion and waste generation compared to palladium. Palladium, while highly efficient in catalytic performance, often involves intensive mining processes that lead to significant ecological disruption and higher carbon emissions. Vanadium's improved sustainability profile supports greener industrial applications, particularly in oxidation and polymerization reactions, where minimizing environmental impact is critical.
Cost and Availability Analysis
Vanadium offers a more cost-effective alternative to palladium in catalyst applications due to its significantly lower market price and wider natural abundance. Palladium prices are highly volatile and driven by limited supply from a few key mining regions, causing higher overall costs and potential supply chain risks. Vanadium's stable availability from diverse global sources supports more consistent pricing and scalability for industrial catalytic processes.
Recent Advances in Catalyst Development
Recent advances in catalyst development reveal vanadium catalysts excel in selective oxidation reactions due to their abundant active sites and cost-effectiveness, enhancing industrial applications such as sulfuric acid production and environmental remediation. Palladium catalysts demonstrate superior performance in hydrogenation and carbon-carbon coupling reactions, benefiting from high activity and stability, particularly in automotive catalytic converters and fine chemical synthesis. Research continues to optimize vanadium and palladium catalytic properties through nanostructuring and alloying, aiming to improve efficiency, selectivity, and resistance to deactivation in sustainable chemical processes.
Challenges and Limitations
Vanadium catalysts face challenges such as low thermal stability and susceptibility to deactivation through sintering and poisoning, limiting their long-term industrial use. Palladium catalysts offer superior activity and selectivity but are constrained by high costs and sensitivity to sulfur and other impurities. Both catalysts encounter limitations in scalability and environmental impact, with vanadium's toxicity and palladium's scarcity requiring careful management in catalytic applications.
Future Prospects for Vanadium and Palladium Catalysts
Vanadium catalysts are gaining traction due to their cost-effectiveness, abundant availability, and robust oxidative properties, making them ideal for large-scale chemical processes such as sulfuric acid production and selective oxidation reactions. Palladium catalysts, known for their exceptional activity and selectivity in hydrogenation and carbon-carbon coupling reactions, continue to dominate high-value applications like pharmaceuticals and automotive catalytic converters despite their higher cost and limited reserves. Future developments in vanadium catalyst technology emphasize enhancing stability and reusability, while palladium research focuses on reducing precious metal content through alloying and nanoparticle engineering to sustain performance and economic viability.
Infographic: Vanadium vs Palladium for Catalyst
 azmater.com
azmater.com