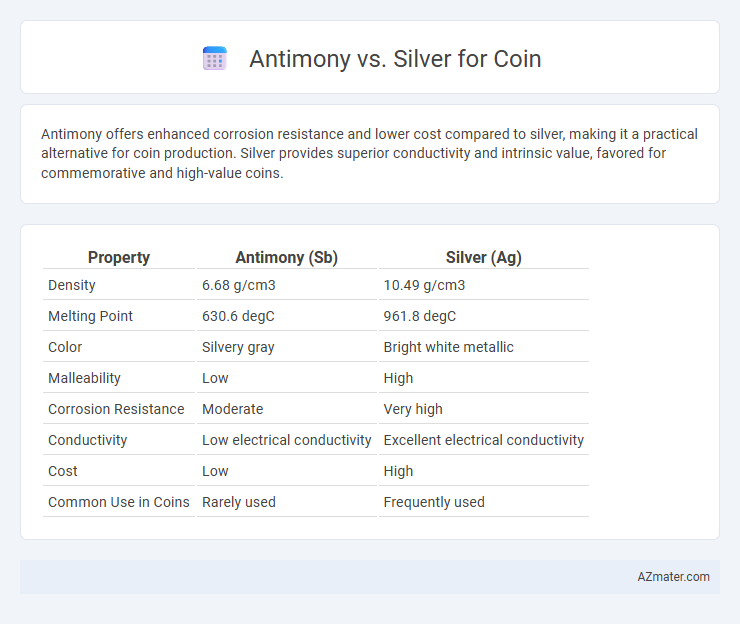
Antimony offers enhanced corrosion resistance and lower cost compared to silver, making it a practical alternative for coin production. Silver provides superior conductivity and intrinsic value, favored for commemorative and high-value coins.
Table of Comparison
| Property | Antimony (Sb) | Silver (Ag) |
|---|---|---|
| Density | 6.68 g/cm3 | 10.49 g/cm3 |
| Melting Point | 630.6 degC | 961.8 degC |
| Color | Silvery gray | Bright white metallic |
| Malleability | Low | High |
| Corrosion Resistance | Moderate | Very high |
| Conductivity | Low electrical conductivity | Excellent electrical conductivity |
| Cost | Low | High |
| Common Use in Coins | Rarely used | Frequently used |
Introduction to Antimony and Silver in Coinage
Antimony and silver both have distinct roles in coinage, with silver being a traditional precious metal widely used for its durability, luster, and intrinsic value in coins and bullion. Antimony, although not commonly used as a primary metal in coins, is valued for its alloying properties, often added to metals like lead to improve hardness and resistance to wear. The use of silver dates back thousands of years as a standard for currency, while antimony's contribution is more specialized, enhancing coin durability when combined with other metals.
Historical Use of Antimony and Silver in Coins
Antimony was rarely used in coinage historically, mainly due to its brittle nature and difficulty in alloying, whereas silver has been a primary metal for coins for millennia due to its durability, luster, and intrinsic value. Ancient civilizations such as the Greeks, Romans, and medieval European societies extensively minted silver coins, including the Roman denarius and the Spanish real, capitalizing on silver's stability and wide acceptance in trade. Antimony's historical significance lies more in metallurgy and alchemy rather than numismatics, making silver the dominant choice for currency through history.
Physical and Chemical Properties Comparison
Antimony and silver differ significantly in physical and chemical properties relevant to coinage. Silver boasts high ductility, malleability, and a melting point of 961.8degC, making it ideal for detailed coin designs and durability. Antimony, with a lower melting point of 630.6degC and brittleness, is less suitable for minting coins due to its hardness, brittleness, and limited resistance to corrosion compared to silver's excellent chemical stability and tarnish resistance.
Durability and Longevity in Circulation
Silver coins demonstrate superior durability and longevity in circulation due to their resistance to corrosion and wear, maintaining their structure and luster over extended periods. Antimony, being a brittle metalloid, is less suitable for coins as it tends to degrade and fracture under mechanical stress, limiting its lifespan. The hardness and malleability of silver contribute to its widespread use in numismatics, ensuring coins remain collectible and functional longer than those made with antimony.
Antimicrobial and Tarnish Resistance
Antimony exhibits significant antimicrobial properties, making it an effective material for reducing bacterial growth on coins, whereas silver's antimicrobial benefits are well-documented but can diminish with surface tarnish. Silver is prone to tarnishing due to sulfide formation, which not only affects its appearance but can also reduce its antimicrobial efficiency over time. In contrast, antimony offers superior tarnish resistance, maintaining both its aesthetic value and antimicrobial performance, making it a more durable choice in environments where hygiene and longevity are priorities.
Economic Value and Market Trends
Silver maintains a higher economic value than antimony, driven by its extensive use in jewelry, electronics, and investment markets, with prices fluctuating around $25 per ounce as of early 2024. Antimony, valued primarily for its industrial applications in flame retardants and lead-acid batteries, commands lower market prices, typically under $10,000 per metric ton, with demand linked to specialized manufacturing needs. Market trends indicate silver remains a stable precious metal with strong investment appeal, while antimony experiences volatile demand influenced by technological advances and geopolitical supply constraints.
Minting Process: Ease of Striking and Casting
Antimony, with its low melting point of about 631degC, offers easier casting compared to silver, which melts at 961.8degC, reducing energy costs and facilitating mass production of coins. However, silver's superior malleability and ductility allow for finer details and sharper reliefs during the striking process, making it preferred for high-quality coinage. The brittleness of antimony can cause challenges in striking, often leading to increased die wear and lower coin durability.
Counterfeiting Risks and Security Features
Silver coins offer intrinsic value and established security features such as intricate minting details and serial numbers, making counterfeiting more difficult compared to antimony coins. Antimony, being less valuable and softer, poses higher counterfeiting risks due to its lower market recognition and ease of replication without advanced security measures. Advanced anti-counterfeiting technologies like micro-engraving and holographic elements are more commonly applied to silver coins, enhancing their reliability and consumer trust.
Environmental and Health Considerations
Antimony, used as an alloy in some coinage, poses environmental risks due to its toxicity and potential to bioaccumulate in ecosystems, causing harm to aquatic life and soil quality during mining and disposal. Silver, while less toxic, still raises environmental concerns related to mining practices that can lead to habitat destruction and water contamination, yet its lower toxicity reduces direct human health risks compared to antimony. Choosing between antimony and silver for coins involves balancing the ecological impact of extraction and toxicity against the durability and value of the metal in circulation.
Conclusion: Choosing the Right Metal for Coinage
Antimony and silver differ significantly in durability, value, and historical use in coinage. Silver's superior corrosion resistance, higher intrinsic value, and widespread acceptance make it the preferred choice for durable, high-value coins, while antimony's brittleness and lower market value limit its suitability for circulating currency. For long-term investment and collector coins, silver remains the optimal metal due to its stability and global demand.
Infographic: Antimony vs Silver for Coin
 azmater.com
azmater.com