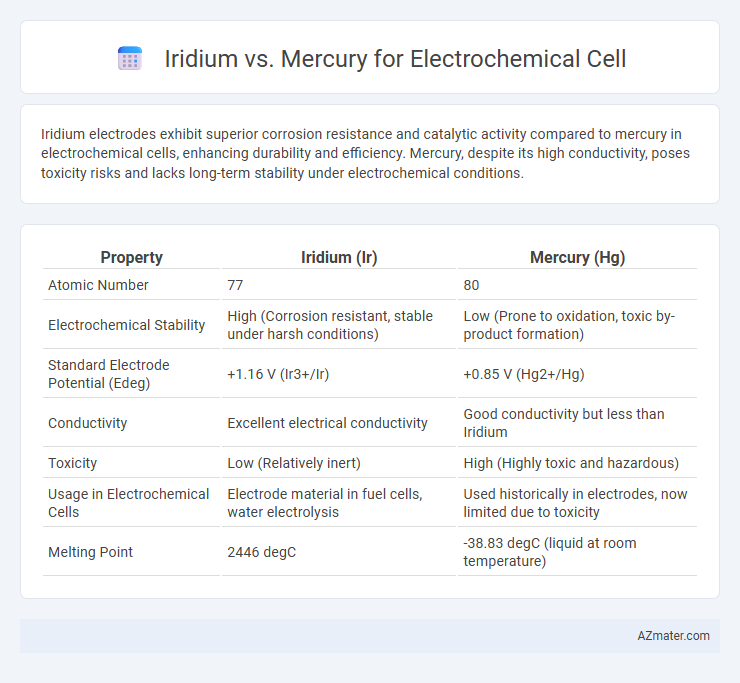
Iridium electrodes exhibit superior corrosion resistance and catalytic activity compared to mercury in electrochemical cells, enhancing durability and efficiency. Mercury, despite its high conductivity, poses toxicity risks and lacks long-term stability under electrochemical conditions.
Table of Comparison
| Property | Iridium (Ir) | Mercury (Hg) |
|---|---|---|
| Atomic Number | 77 | 80 |
| Electrochemical Stability | High (Corrosion resistant, stable under harsh conditions) | Low (Prone to oxidation, toxic by-product formation) |
| Standard Electrode Potential (Edeg) | +1.16 V (Ir3+/Ir) | +0.85 V (Hg2+/Hg) |
| Conductivity | Excellent electrical conductivity | Good conductivity but less than Iridium |
| Toxicity | Low (Relatively inert) | High (Highly toxic and hazardous) |
| Usage in Electrochemical Cells | Electrode material in fuel cells, water electrolysis | Used historically in electrodes, now limited due to toxicity |
| Melting Point | 2446 degC | -38.83 degC (liquid at room temperature) |
Introduction to Electrochemical Cells
Iridium and mercury serve distinct roles in electrochemical cells due to their unique electrochemical properties. Iridium, known for its high corrosion resistance and catalytic activity, is often utilized as an electrode material in oxygen evolution reactions, enhancing cell efficiency and durability. Mercury, historically used for its stable liquid state and excellent electrical conductivity, provides a reproducible electrode surface but poses toxicity and environmental concerns limiting its modern application.
Overview of Iridium in Electrochemistry
Iridium exhibits exceptional stability and catalytic activity in electrochemical cells, especially under harsh oxidative conditions, making it a preferred choice for oxygen evolution reactions (OER) in water splitting processes. Its high corrosion resistance and ability to maintain conductive properties at elevated potentials outperform mercury, which is toxic and less stable in electrochemical environments. These attributes position iridium as a critical material in advanced electrochemical applications, including fuel cells and electrolyzers.
Overview of Mercury in Electrochemistry
Mercury serves as a key electrode material in electrochemical cells due to its high electrical conductivity and unique ability to form amalgams with various metals, facilitating distinct electrochemical reactions. It exhibits a stable electrochemical potential, making it ideal for reference electrodes like the calomel electrode, which is widely used for precise voltage measurements. Despite its toxic nature, mercury's liquid state at room temperature allows for smooth electrode surfaces and reproducible electrochemical behavior, critical for accurate and sensitive analyses.
Key Physical and Chemical Properties
Iridium exhibits exceptional corrosion resistance and high catalytic activity, making it ideal for electrochemical cells operating under harsh conditions and at elevated temperatures, whereas mercury's liquid state at room temperature ensures excellent conductivity but poses toxicity concerns and limited chemical stability. Iridium's high melting point (2446degC) and strong oxidation resistance contrast sharply with mercury's low melting point (-38.83degC) and vulnerability to oxidation and amalgamation reactions. The inertness of iridium electrodes supports prolonged durability in electrochemical applications, while mercury electrodes are often favored for their unique voltammetric properties but require careful handling due to environmental and health risks.
Electrode Performance: Iridium vs Mercury
Iridium electrodes exhibit superior electrochemical stability and corrosion resistance compared to mercury, enabling prolonged durability in harsh electrochemical cell environments. The catalytic activity of iridium for oxygen evolution reactions significantly surpasses that of mercury, resulting in enhanced reaction rates and energy efficiency. Mercury electrodes, while historically used for their liquid state and wide potential window, suffer from toxicity and limited electrochemical robustness, making iridium a preferred choice in modern electrochemical applications.
Toxicity and Environmental Impact
Iridium exhibits significantly lower toxicity compared to mercury, making it a safer choice for electrochemical cells in terms of environmental health. Mercury is highly toxic, posing severe risks to aquatic ecosystems and bioaccumulating in the food chain, which leads to serious environmental contamination. The use of iridium reduces hazardous waste generation and aligns with stricter environmental regulations aimed at minimizing heavy metal pollution.
Cost and Availability Comparison
Iridium and mercury differ significantly in cost and availability for electrochemical cells, with iridium being a rare and expensive noble metal, leading to higher material costs and limited supply. Mercury is more abundant and relatively inexpensive, but its toxicity and environmental regulations restrict its use. Choosing between iridium and mercury depends on balancing the high cost and scarcity of iridium against the regulatory risks and lower expense of mercury.
Applications in Modern Electrochemical Devices
Iridium electrodes exhibit superior corrosion resistance and catalytic activity, making them ideal for proton exchange membrane (PEM) electrolyzers and electrochemical sensors in harsh environments. Mercury, known for its high electrical conductivity and liquid state at room temperature, is primarily used in reference electrodes and specialized analytical electrochemical cells. Modern electrochemical devices favor iridium for durability and efficiency, while mercury remains limited due to toxicity concerns and environmental regulations.
Safety Considerations and Handling Procedures
Iridium electrodes in electrochemical cells offer superior corrosion resistance and lower toxicity compared to mercury, reducing health hazards during handling and disposal. Mercury poses significant safety risks due to its toxicity and vapor inhalation potential, requiring stringent containment and specialized PPE such as gloves and fume hoods. Proper handling procedures for iridium involve standard laboratory protocols, while mercury demands rigorous spill response plans and environmental regulations to mitigate contamination.
Future Trends and Alternatives in Electrode Materials
Iridium remains a leading electrode material in electrochemical cells due to its high corrosion resistance and catalytic activity, essential for oxygen evolution reactions, yet its scarcity and cost drive the search for alternatives. Emerging materials like transition metal oxides, including doped manganese and cobalt oxides, show promising electrochemical performance with improved sustainability and lower expense. Future trends emphasize developing earth-abundant, durable electrode materials with enhanced conductivity and stability to replace iridium, enabling scalable and cost-effective electrochemical energy conversion technologies.
Infographic: Iridium vs Mercury for Electrochemical Cell
 azmater.com
azmater.com