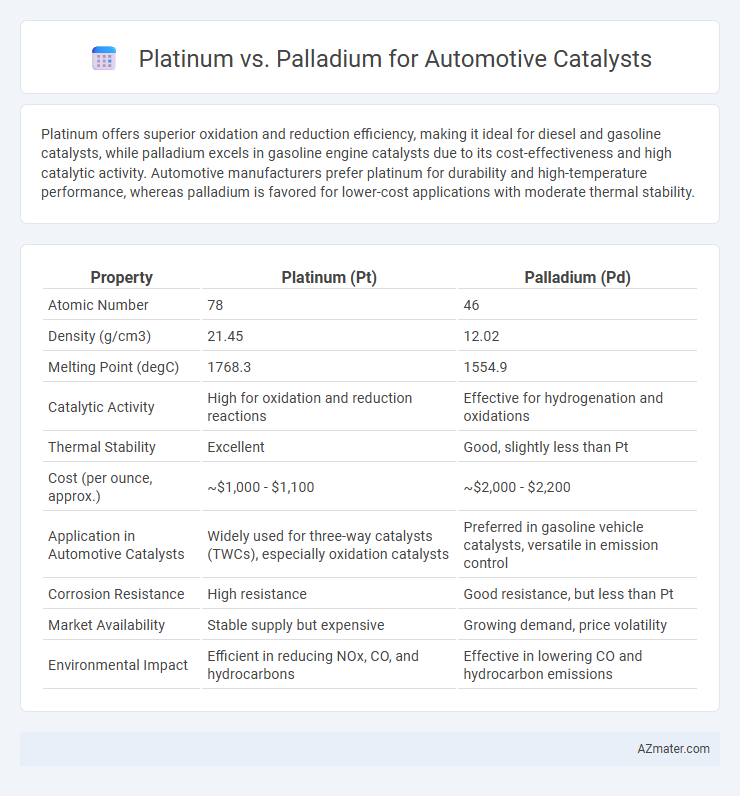
Platinum offers superior oxidation and reduction efficiency, making it ideal for diesel and gasoline catalysts, while palladium excels in gasoline engine catalysts due to its cost-effectiveness and high catalytic activity. Automotive manufacturers prefer platinum for durability and high-temperature performance, whereas palladium is favored for lower-cost applications with moderate thermal stability.
Table of Comparison
| Property | Platinum (Pt) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 78 | 46 |
| Density (g/cm3) | 21.45 | 12.02 |
| Melting Point (degC) | 1768.3 | 1554.9 |
| Catalytic Activity | High for oxidation and reduction reactions | Effective for hydrogenation and oxidations |
| Thermal Stability | Excellent | Good, slightly less than Pt |
| Cost (per ounce, approx.) | ~$1,000 - $1,100 | ~$2,000 - $2,200 |
| Application in Automotive Catalysts | Widely used for three-way catalysts (TWCs), especially oxidation catalysts | Preferred in gasoline vehicle catalysts, versatile in emission control |
| Corrosion Resistance | High resistance | Good resistance, but less than Pt |
| Market Availability | Stable supply but expensive | Growing demand, price volatility |
| Environmental Impact | Efficient in reducing NOx, CO, and hydrocarbons | Effective in lowering CO and hydrocarbon emissions |
Overview: Platinum and Palladium in Automotive Catalysts
Platinum and palladium are critical metals in automotive catalysts, primarily used to reduce harmful emissions from vehicle exhaust systems. Platinum excels in oxidation reactions, converting carbon monoxide and hydrocarbons into less harmful substances, while palladium is highly effective in oxidizing pollutants under lean-burn conditions. The choice between platinum and palladium in catalytic converters depends on factors like fuel type, emission standards, and cost, with palladium often preferred in gasoline engines due to its lower price and high activity.
Chemical Properties and Behavior
Platinum exhibits exceptional catalytic activity due to its high electron affinity and stability at elevated temperatures, making it ideal for oxidation reactions in automotive catalysts. Palladium, with a slightly lower melting point and greater flexibility in alloy formation, demonstrates superior performance in hydrocarbon and carbon monoxide conversion under fluctuating engine conditions. Both metals resist poisoning by sulfur compounds, but platinum maintains longer durability in harsh exhaust environments, influencing catalyst longevity and efficiency.
Historical Use in Automotive Industry
Platinum has been a dominant catalyst metal in automotive catalytic converters since the 1970s due to its high efficiency in oxidizing carbon monoxide and hydrocarbons. Palladium gained prominence in the 1980s and 1990s as a cost-effective alternative with strong performance in reducing nitrogen oxides and hydrocarbons, especially in gasoline engines. The automotive industry continues to balance the use of platinum and palladium based on fuel type, emissions standards, and material costs, with palladium increasingly favored in modern gasoline catalytic converters.
Catalytic Efficiency and Performance
Platinum exhibits superior catalytic efficiency in automotive catalysts due to its high resistance to poisoning and excellent durability under high-temperature conditions, making it highly effective for oxidizing carbon monoxide and hydrocarbons. Palladium offers enhanced catalytic performance in lean-burn engines by promoting efficient nitrogen oxide reduction and displaying greater activity at lower temperatures. The choice between platinum and palladium directly impacts emissions control efficacy and overall catalyst longevity in various engine types.
Cost Comparison and Market Trends
Platinum and palladium are critical metals used in automotive catalysts, with palladium currently experiencing higher market prices due to increased demand in gasoline vehicle emission controls. Cost comparison reveals palladium is generally more expensive than platinum, driven by supply constraints and geopolitical factors affecting mining regions like Russia and South Africa. Market trends indicate a potential shift back to platinum as automakers seek cost-effective alternatives amid tightening emission regulations and volatile palladium prices.
Availability and Global Supply Chains
Platinum and palladium are critical metals used in automotive catalysts, with palladium currently facing more supply constraints due to its limited production primarily concentrated in Russia and South Africa. Platinum enjoys a relatively more stable availability, supported by diversified mining operations and growing recycling efforts, which stabilize its global supply chains. Disruptions in Russia's exports or geopolitical tensions can drastically impact palladium's availability, making platinum a strategic alternative in automotive catalytic applications.
Environmental Impact and Sustainability
Platinum and palladium serve critical roles in automotive catalysts by reducing harmful emissions, with palladium favored for gasoline engines due to its superior oxidation capabilities, while platinum excels in diesel catalysts for nitrogen oxide reduction. Palladium's increasing demand raises concerns about resource scarcity and environmental impact during mining, while platinum, though rarer, is more recyclable, enhancing sustainability prospects. Innovations in catalyst technology aim to optimize metal usage and improve recycling processes, minimizing ecological footprints and supporting long-term resource sustainability in the automotive industry.
Resistance to Poisoning and Durability
Platinum exhibits superior resistance to poisoning by sulfur and lead contaminants in automotive catalysts, maintaining high catalytic activity under harsh operating conditions. Palladium, while generally less costly, tends to degrade faster due to its lower tolerance to poisons and thermal aging, affecting long-term durability. Consequently, platinum-based catalysts offer enhanced stability and longer service life in exhaust emission control systems compared to palladium-based counterparts.
Future Prospects and Technological Innovations
Platinum and palladium remain critical in automotive catalyst technologies, with platinum gaining prominence due to its superior durability and efficiency in high-temperature conditions, crucial for evolving emissions standards. Advances in catalyst formulation, including nano-engineered platinum particles and hybrid catalysts combining platinum and palladium, improve conversion rates and reduce reliance on scarce materials. Emerging trends in electric and hybrid vehicles drive research towards optimizing platinum group metals for improved catalytic performance and cost-effective production, ensuring sustainable growth in future automotive applications.
Choosing the Right Metal for Automotive Applications
Platinum and palladium are critical metals used in automotive catalysts for reducing harmful emissions, with palladium favored for gasoline engines due to its superior oxidation capabilities and cost-effectiveness. Platinum excels in diesel catalysts because of its higher durability and better performance in high-temperature environments. Selecting the right metal depends on engine type, operating conditions, and environmental regulations, ensuring optimal catalyst efficiency and compliance.
Infographic: Platinum vs Palladium for Automotive Catalyst
 azmater.com
azmater.com