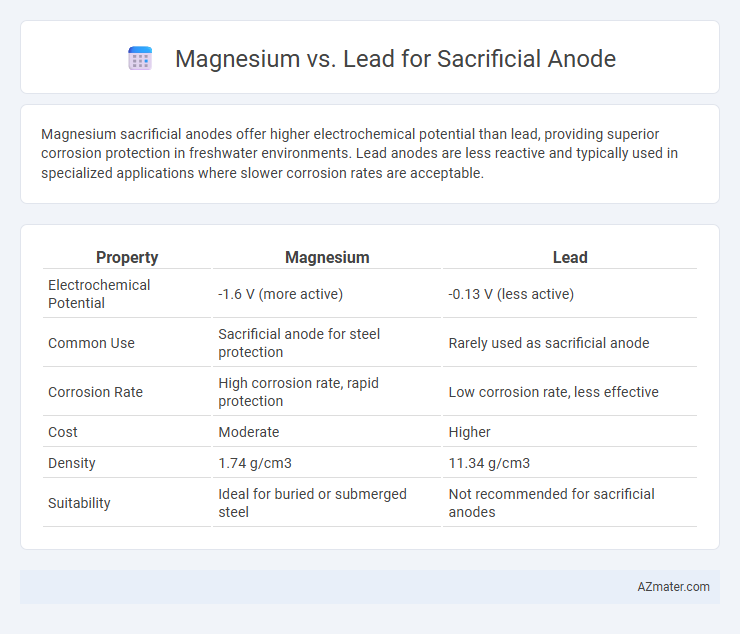
Magnesium sacrificial anodes offer higher electrochemical potential than lead, providing superior corrosion protection in freshwater environments. Lead anodes are less reactive and typically used in specialized applications where slower corrosion rates are acceptable.
Table of Comparison
| Property | Magnesium | Lead |
|---|---|---|
| Electrochemical Potential | -1.6 V (more active) | -0.13 V (less active) |
| Common Use | Sacrificial anode for steel protection | Rarely used as sacrificial anode |
| Corrosion Rate | High corrosion rate, rapid protection | Low corrosion rate, less effective |
| Cost | Moderate | Higher |
| Density | 1.74 g/cm3 | 11.34 g/cm3 |
| Suitability | Ideal for buried or submerged steel | Not recommended for sacrificial anodes |
Introduction to Sacrificial Anodes
Sacrificial anodes protect metal structures from corrosion by corroding themselves instead of the protected metal. Magnesium anodes offer higher driving voltage, making them suitable for low-resistance environments such as soil and freshwater, whereas lead anodes are less common due to lower electrochemical activity. The choice between magnesium and lead depends on the conductivity of the environment and the specific cathodic protection requirements.
The Role of Sacrificial Anodes in Corrosion Protection
Magnesium sacrificial anodes offer a higher electrochemical potential than lead, making them more effective in protecting steel structures in low-resistivity environments such as soil or freshwater. Lead anodes, while less reactive, provide longer-lasting protection in specific conditions with higher resistivity but are less commonly used due to environmental concerns. The choice between magnesium and lead anodes directly impacts the efficiency of corrosion prevention by determining the rate and duration of metal ion sacrifice to shield the underlying structure.
Chemical Properties: Magnesium vs. Lead
Magnesium exhibits a more negative electrochemical potential (-2.37 V) compared to lead (-0.13 V), making it significantly more reactive and effective as a sacrificial anode in protecting steel structures from corrosion. The high activity of magnesium allows it to oxidize preferentially, sacrificing itself to prevent the oxidation of the protected metal, whereas lead's lower reactivity limits its use primarily to specific conditions with less aggressive environments. Magnesium's lightweight and ample availability further enhance its suitability for marine and underground applications, while lead's toxicity restricts its use in environmentally sensitive areas.
Electrochemical Potential Comparison
Magnesium exhibits a more negative electrochemical potential (-2.37 V) compared to lead (-0.13 V) on the standard hydrogen electrode scale, making it a more effective sacrificial anode for corrosion protection. The higher driving force from magnesium's potential enables it to preferentially oxidize, protecting the underlying metal structure longer than lead. Consequently, magnesium anodes are widely preferred in environments requiring aggressive corrosion resistance, whereas lead's milder potential limits its sacrificial capability.
Efficiency in Various Environments
Magnesium anodes exhibit higher efficiency in low-resistivity environments such as freshwater due to their lower electrode potential, providing superior corrosion protection for steel structures. Lead anodes, while less common for sacrificial use, offer stability and longevity in high-resistivity or alkaline environments but typically generate lower current output. Selecting magnesium over lead improves protection efficiency in marine and freshwater applications where rapid and effective cathodic protection is required.
Longevity and Maintenance Requirements
Magnesium sacrificial anodes offer superior longevity due to their higher electrochemical potential, making them ideal for environments with low electrical conductivity such as freshwater systems. Lead anodes, while less common, require more frequent maintenance because of their lower corrosion efficiency and shorter lifespan. Choosing magnesium anodes reduces overall maintenance costs and extends service intervals in cathodic protection applications.
Environmental Impact and Safety Considerations
Magnesium sacrificial anodes, widely used for their high electrochemical potential, degrade into non-toxic byproducts, making them environmentally safer compared to lead anodes, which can release harmful lead ions into surrounding ecosystems. Lead anodes pose significant health and environmental risks due to lead's toxicity, necessitating stringent handling and disposal procedures to prevent soil and water contamination. Magnesium's lower environmental impact and safer degradation profile make it the preferred choice where ecological safety is paramount.
Cost Effectiveness of Magnesium and Lead Anodes
Magnesium sacrificial anodes offer superior cost effectiveness due to their higher driving voltage and faster corrosion rate, which provides better protection for steel structures in low resistivity environments. Lead anodes, while more durable and slower to deplete, often have higher initial costs and require more maintenance, making them less economical for large-scale or long-term cathodic protection projects. The lower price per ampere-hour of magnesium anodes generally results in reduced overall system costs and easier replacement cycles.
Common Applications for Magnesium and Lead Anodes
Magnesium anodes are predominantly used in freshwater environments and soil due to their high driving voltage and rapid corrosion rate, making them ideal for protecting underground tanks, pipelines, and water heaters. Lead anodes, though less common, are primarily utilized in specialized industrial electroplating processes and as cathodic protection in environments where low current output is sufficient, such as certain marine applications. The choice between magnesium and lead sacrificial anodes depends on the electrolyte resistivity and the required current output to effectively prevent metal corrosion.
Choosing the Right Sacrificial Anode: Key Factors
Choosing the right sacrificial anode involves evaluating magnesium and lead based on factors like electrochemical potential, environmental conditions, and application type. Magnesium anodes provide higher driving voltage, making them ideal for freshwater and low conductivity environments, while lead anodes are suited for marine or high salinity conditions due to their stability and lower potential for hydrogen embrittlement. Corrosion rate, cost-effectiveness, and compatibility with the metal structure's characteristics also influence the optimal choice for long-term protection.
Infographic: Magnesium vs Lead for Sacrificial Anode
 azmater.com
azmater.com