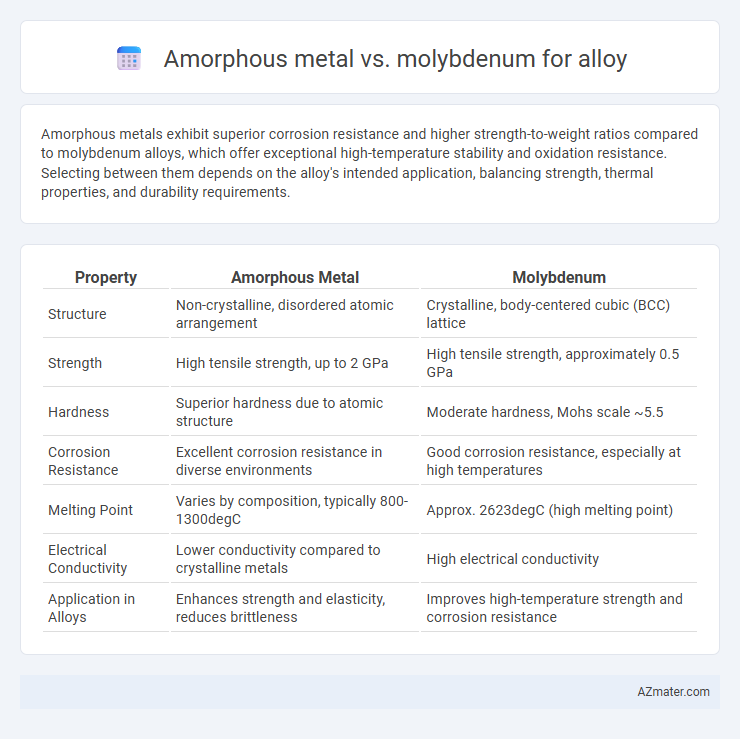
Amorphous metals exhibit superior corrosion resistance and higher strength-to-weight ratios compared to molybdenum alloys, which offer exceptional high-temperature stability and oxidation resistance. Selecting between them depends on the alloy's intended application, balancing strength, thermal properties, and durability requirements.
Table of Comparison
| Property | Amorphous Metal | Molybdenum |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Crystalline, body-centered cubic (BCC) lattice |
| Strength | High tensile strength, up to 2 GPa | High tensile strength, approximately 0.5 GPa |
| Hardness | Superior hardness due to atomic structure | Moderate hardness, Mohs scale ~5.5 |
| Corrosion Resistance | Excellent corrosion resistance in diverse environments | Good corrosion resistance, especially at high temperatures |
| Melting Point | Varies by composition, typically 800-1300degC | Approx. 2623degC (high melting point) |
| Electrical Conductivity | Lower conductivity compared to crystalline metals | High electrical conductivity |
| Application in Alloys | Enhances strength and elasticity, reduces brittleness | Improves high-temperature strength and corrosion resistance |
Introduction to Amorphous Metals and Molybdenum Alloys
Amorphous metals, also known as metallic glasses, are characterized by their non-crystalline atomic structure, offering superior strength, corrosion resistance, and elasticity compared to conventional crystalline metals. Molybdenum alloys are valued for their high melting point, excellent thermal conductivity, and strong resistance to wear and corrosion, making them ideal for high-temperature and structural applications. Comparing these materials highlights amorphous metals' advantages in mechanical performance and molybdenum alloys' reliability in extreme environments.
Structural Composition and Atomic Arrangement
Amorphous metals exhibit a non-crystalline atomic arrangement with a random, short-range order, which enhances strength and corrosion resistance by eliminating grain boundaries present in traditional alloys like molybdenum. Molybdenum, a refractory metal, possesses a body-centered cubic (BCC) crystal structure that provides high melting points and excellent structural stability, making it suitable for high-temperature applications. The atomic arrangement differences between amorphous metals and molybdenum critically influence their mechanical properties, with amorphous metals offering superior elasticity and hardness compared to the more ductile and thermally stable crystalline molybdenum alloys.
Mechanical Strength and Hardness Comparison
Amorphous metals exhibit superior mechanical strength and hardness compared to molybdenum alloys due to their non-crystalline atomic structure, which eliminates grain boundaries and enhances resistance to deformation. Molybdenum alloys, while possessing high melting points and good tensile strength, generally have lower hardness and are more susceptible to fracture under stress. The unique atomic arrangement in amorphous metals results in higher yield strength and hardness values, making them preferable for applications requiring extreme wear resistance and mechanical durability.
Corrosion and Oxidation Resistance
Amorphous metals exhibit superior corrosion resistance compared to molybdenum-based alloys due to their non-crystalline, homogeneous atomic structure that eliminates grain boundaries prone to corrosion. Molybdenum enhances oxidation resistance in alloys by forming stable, protective oxide layers at high temperatures, but these layers can degrade under harsh chemical environments. Combining amorphous metal coatings with molybdenum-containing substrates can optimize overall resistance to both corrosion and oxidation in demanding industrial applications.
Electrical and Thermal Conductivity Differences
Amorphous metals exhibit significantly lower electrical and thermal conductivity compared to molybdenum due to their disordered atomic structure, which scatters electrons and phonons more effectively. Molybdenum, a crystalline transition metal, boasts high electrical conductivity around 1.9 x 10^7 S/m and thermal conductivity approximately 138 W/m*K, making it efficient for heat and current transfer. Alloy applications requiring superior conductivity often favor molybdenum over amorphous metals, despite the latter's advantages in mechanical strength and corrosion resistance.
Magnetic Properties of Amorphous Metals vs Molybdenum
Amorphous metals exhibit superior soft magnetic properties characterized by low coercivity and high magnetic permeability due to their non-crystalline atomic structure, which reduces magnetic domain wall pinning. In contrast, molybdenum, a crystalline transition metal, has limited magnetic behavior as it is paramagnetic with relatively low magnetic susceptibility. The unique disordered structure of amorphous metals enables enhanced magnetic performance in transformer cores and inductors, where molybdenum's application is more structural than magnetic.
Weight, Density, and Alloy Design Considerations
Amorphous metals typically exhibit lower density than molybdenum, making them advantageous for lightweight alloy applications where strength-to-weight ratio is critical. Molybdenum's high density (10.28 g/cm3) and exceptional high-temperature strength require designers to balance weight with durability in alloy formulations. Alloy design considerations favor amorphous metals for corrosion resistance and elasticity, while molybdenum alloys prioritize thermal stability and wear resistance in demanding environments.
Cost, Availability, and Manufacturing Challenges
Amorphous metals, also known as metallic glasses, generally incur higher production costs than molybdenum due to complex cooling processes required to achieve their non-crystalline structure. Molybdenum boasts greater availability as a naturally abundant refractory metal with established extraction and refining methods, making it more cost-effective for alloy manufacturing. Manufacturing challenges with amorphous metals include maintaining precise cooling rates to prevent crystallization, whereas molybdenum's high melting point and brittleness demand specialized equipment and techniques during alloy fabrication.
Typical Industrial and Technological Applications
Amorphous metals, also known as metallic glasses, exhibit exceptional strength, corrosion resistance, and magnetic properties, making them ideal for applications in transformer cores, sporting goods, and precision medical instruments. Molybdenum alloys are prized for their high melting point, excellent thermal conductivity, and resistance to wear and corrosion, extensively used in aerospace components, electrical contacts, and high-temperature furnace parts. While amorphous metals excel in magnetic and wear-sensitive sectors, molybdenum alloys are preferred for structural and thermal resilience in extreme industrial environments.
Future Trends in Advanced Alloy Development
Amorphous metals exhibit exceptional strength and corrosion resistance due to their non-crystalline atomic structure, making them ideal for future alloy development focused on lightweight and durable materials. Molybdenum, known for its high melting point and excellent thermal stability, remains critical in alloys for aerospace and energy applications that demand extreme temperature endurance. Advances in additive manufacturing and nanoscale alloying techniques are driving innovations that combine amorphous metals with molybdenum to create hybrid materials with enhanced mechanical and thermal properties.
Infographic: Amorphous metal vs Molybdenum for Alloy
 azmater.com
azmater.com