Osmium, with its exceptional density and stability, is ideal for advanced material science and catalysis research, while Fermium, a highly radioactive synthetic element, is primarily used in nuclear chemistry and advanced nuclear physics studies. Researchers prioritize Osmium for practical applications due to its availability and durability, whereas Fermium's rarity and radioactivity limit its use to specialized experimental setups.
Table of Comparison
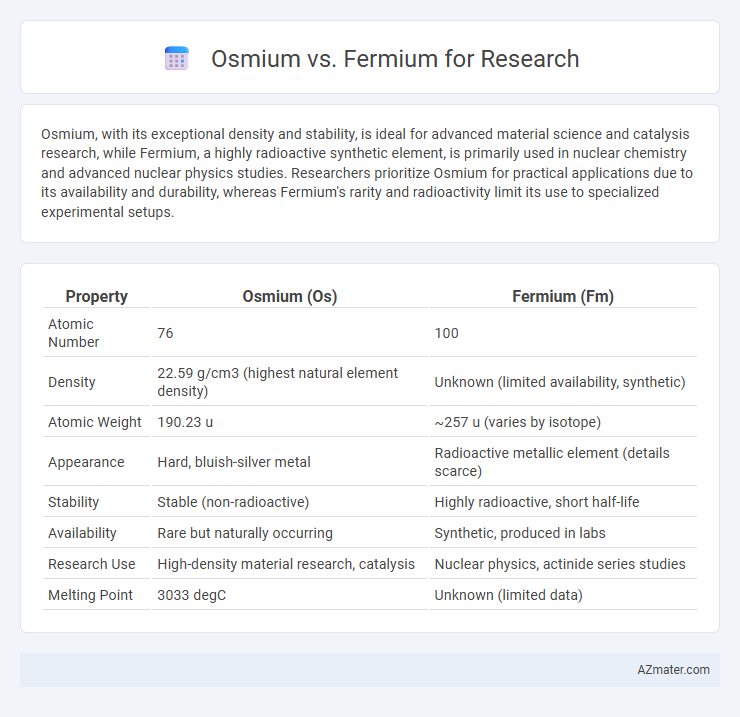
| Property | Osmium (Os) | Fermium (Fm) |
|---|---|---|
| Atomic Number | 76 | 100 |
| Density | 22.59 g/cm3 (highest natural element density) | Unknown (limited availability, synthetic) |
| Atomic Weight | 190.23 u | ~257 u (varies by isotope) |
| Appearance | Hard, bluish-silver metal | Radioactive metallic element (details scarce) |
| Stability | Stable (non-radioactive) | Highly radioactive, short half-life |
| Availability | Rare but naturally occurring | Synthetic, produced in labs |
| Research Use | High-density material research, catalysis | Nuclear physics, actinide series studies |
| Melting Point | 3033 degC | Unknown (limited data) |
Introduction to Osmium and Fermium
Osmium, a dense transition metal with atomic number 76, is prized for its hardness, high melting point, and significant applications in catalysis and electrical contacts. Fermium, a synthetic actinide element with atomic number 100, is primarily of interest in nuclear research due to its radioactive properties and limited availability. The contrasting natural abundance and chemical behavior of osmium and fermium significantly influence their roles and challenges in scientific investigations.
Atomic Structure and Properties
Osmium, with atomic number 76, is a dense transition metal known for its high atomic mass and remarkable hardness, making it invaluable in applications requiring durability and corrosion resistance. Fermium, atomic number 100, is a synthetic actinide element with a highly unstable nucleus and limited availability, restricting its use primarily to nuclear research and studies of heavy element behaviors. The contrasting atomic structures--Osmium's stable electron configuration versus Fermium's radioactive decay modes--define their distinct roles in scientific investigations and material science.
Natural Occurrence and Production Methods
Osmium, a naturally occurring element found in platinum ores with an abundance of about 1 part per 10 million in Earth's crust, is primarily obtained through mining and complex refining processes. Fermium, a synthetic element not found naturally, is produced exclusively in particle accelerators or nuclear reactors via neutron capture reactions on lighter actinides. The extensive natural availability of osmium contrasts with fermium's reliance on highly specialized nuclear synthesis methods for its generation.
Chemical Reactivity and Stability
Osmium exhibits exceptional chemical stability with a high melting point of 3045degC and limited reactivity, making it suitable for catalysis and long-term applications in harsh environments. Fermium, as a synthetic actinide with a half-life of approximately 100.5 days for its most stable isotope ^257Fm, is highly radioactive and exhibits rapid decay, limiting its practical chemical reactivity studies. The distinct stability and radioactivity profiles of osmium and fermium directly influence their usability in chemical research, with osmium being favored for stable catalytic processes and fermium for nuclear and radiochemical investigations.
Applications in Scientific Research
Osmium and fermium serve distinct roles in scientific research due to their unique properties and availability. Osmium, a dense transition metal, is widely used in electron microscopy for staining biological samples and as a catalyst in chemical reactions, enhancing precision in materials science and biology. Fermium, a synthetic actinide produced in nuclear reactors, is primarily utilized in nuclear chemistry and physics research to study heavy element synthesis and nuclear reactions, contributing to advancements in understanding atomic behavior and nuclear stability.
Handling and Safety Considerations
Osmium, a dense transition metal, requires careful handling due to its toxic osmium tetroxide vapor, which can cause severe respiratory issues and skin irritation; therefore, work must be conducted in well-ventilated fume hoods with appropriate personal protective equipment (PPE). Fermium, a synthetic, highly radioactive actinide produced in minuscule quantities, demands stringent radiological safety measures including glove boxes, remote handling tools, and specialized containment to prevent radiation exposure and contamination. Researchers prioritize minimizing direct contact with osmium compounds and employ rigorous monitoring protocols when working with fermium to maintain laboratory safety and compliance with regulatory standards.
Cost and Availability for Researchers
Osmium, a dense transition metal, is relatively more available and affordable compared to Fermium, a synthetic element produced in minute quantities via nuclear reactions. Researchers face significant cost barriers with Fermium due to its scarce production in specialized facilities, limiting its accessibility for experimental studies. Osmium's greater availability and lower cost make it a more practical choice for extensive research applications requiring heavy metals.
Analytical Techniques for Study
Osmium and Fermium offer distinct analytical challenges and opportunities in research, with osmium often studied using inductively coupled plasma mass spectrometry (ICP-MS) for its precise isotopic analysis and electron microprobe for spatial distribution in geological samples. Fermium, a synthetic actinide, requires advanced radiochemical separation techniques paired with alpha spectroscopy and accelerator mass spectrometry (AMS) to study its nuclear properties and decay pathways. High-resolution transmission electron microscopy (HRTEM) also aids in characterizing these elements at the atomic scale, crucial for understanding their electronic structures and reactivity.
Comparative Advantages in Research
Osmium offers exceptional density and corrosion resistance, making it ideal for high-precision instrument components and advanced materials research. Fermium, a synthetic element with high radioactivity and short half-life, is primarily utilized in nuclear science for studying heavy element synthesis and radioactive decay processes. The comparative advantage lies in Osmium's stability and practical applications in materials science, whereas Fermium's value resides in fundamental nuclear research despite its scarcity and instability.
Future Prospects in Advanced Studies
Osmium's exceptional density and corrosion resistance position it as a critical material in nanotechnology and catalysis research, offering promising applications in advanced electronics and chemical synthesis. Fermium, a synthetic element with high atomic number and radioactivity, remains primarily valuable for nuclear physics studies and the investigation of heavy element behavior, despite its limited availability and short half-life. Future research is likely to explore osmium's role in developing ultra-durable materials, while fermium's prospects focus on expanding the understanding of superheavy element properties and nuclear reactions.
Infographic: Osmium vs Fermium for Research
 azmater.com
azmater.com