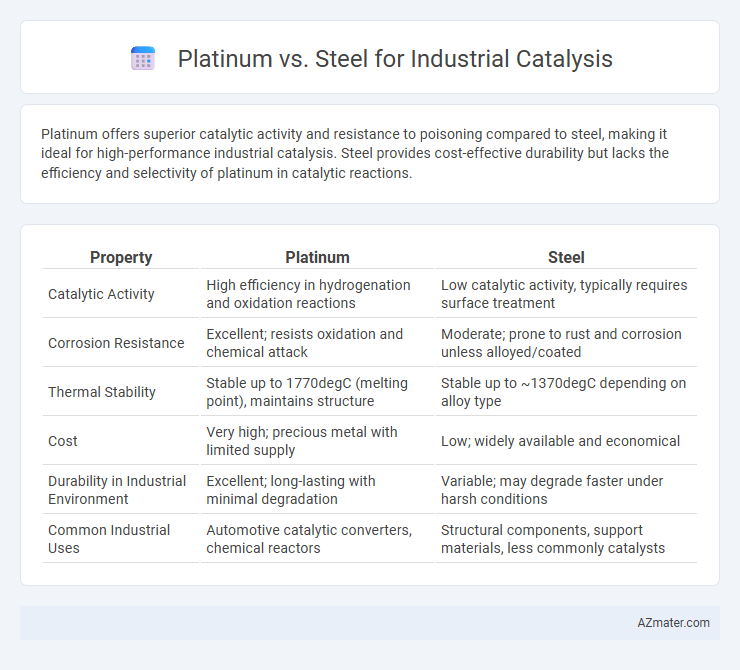
Platinum offers superior catalytic activity and resistance to poisoning compared to steel, making it ideal for high-performance industrial catalysis. Steel provides cost-effective durability but lacks the efficiency and selectivity of platinum in catalytic reactions.
Table of Comparison
| Property | Platinum | Steel |
|---|---|---|
| Catalytic Activity | High efficiency in hydrogenation and oxidation reactions | Low catalytic activity, typically requires surface treatment |
| Corrosion Resistance | Excellent; resists oxidation and chemical attack | Moderate; prone to rust and corrosion unless alloyed/coated |
| Thermal Stability | Stable up to 1770degC (melting point), maintains structure | Stable up to ~1370degC depending on alloy type |
| Cost | Very high; precious metal with limited supply | Low; widely available and economical |
| Durability in Industrial Environment | Excellent; long-lasting with minimal degradation | Variable; may degrade faster under harsh conditions |
| Common Industrial Uses | Automotive catalytic converters, chemical reactors | Structural components, support materials, less commonly catalysts |
Introduction to Industrial Catalysis
Platinum and steel serve distinct roles in industrial catalysis, where platinum acts as a highly efficient noble metal catalyst due to its excellent chemical stability and superior catalytic activity in processes like hydrogenation and oxidation. Steel, often used as a support material or structural component, provides mechanical strength and cost-effective durability but lacks the intrinsic catalytic properties of platinum. The choice between platinum and steel hinges on balancing catalytic performance with economic and operational factors in large-scale industrial applications.
The Role of Platinum in Catalytic Processes
Platinum plays a critical role in industrial catalysis due to its exceptional catalytic activity, high resistance to poisoning, and ability to facilitate hydrogenation, oxidation, and reforming reactions. Its unique electronic structure enables effective adsorption and activation of reactant molecules, leading to enhanced reaction rates compared to steel-based catalysts. Unlike steel, platinum's superior stability under high temperatures and corrosive environments makes it indispensable for catalytic converters, fuel cells, and chemical synthesis processes.
Steel as a Catalyst: Capabilities and Limitations
Steel, primarily composed of iron with carbon and alloying elements, offers cost-effective catalytic properties in industrial processes such as ammonia synthesis and Fischer-Tropsch reactions. Its catalytic performance is often limited by lower activity and selectivity compared to platinum, requiring higher temperatures and promoting coke formation, which can deactivate the catalyst. Advances in steel alloying and surface treatments aim to enhance durability and resistance to sintering, yet steel catalysts generally remain less efficient than platinum in critical applications demanding high precision and longevity.
Cost Comparison: Platinum vs Steel
Platinum's high catalytic efficiency comes at a significant cost, with prices often exceeding thousands of dollars per ounce compared to steel, which remains relatively inexpensive and abundant. Steel's affordability and mechanical robustness make it a preferred choice for large-scale industrial catalysis applications where cost-efficiency is critical. However, the superior catalytic performance and resistance to corrosion of platinum can justify its higher initial investment in specialized, high-value catalytic processes.
Catalytic Efficiency and Activity
Platinum exhibits superior catalytic efficiency and activity compared to steel due to its excellent ability to adsorb and activate reactant molecules, facilitating faster reaction rates in industrial catalysis. Its high resistance to oxidation and corrosion ensures prolonged catalytic performance under harsh reaction conditions, unlike steel which suffers from surface degradation and lower catalytic activity. The unique electronic structure of platinum enhances its selectivity and turnover frequency, making it the preferred choice for critical catalytic processes such as hydrogenation and reforming.
Durability and Longevity of Catalysts
Platinum catalysts exhibit superior durability and longevity compared to steel counterparts due to their resistance to corrosion and high-temperature stability, essential for industrial catalytic processes. Steel catalysts often suffer from oxidation and mechanical degradation under harsh reaction conditions, limiting their operational lifespan. The enhanced stability of platinum catalysts significantly reduces maintenance costs and downtime, improving overall process efficiency in industrial applications.
Environmental Impact and Sustainability
Platinum catalysts exhibit superior activity and selectivity but pose higher environmental risks due to mining's energy intensity and scarcity, leading to greater ecological footprints. Steel-based catalysts, while generally less efficient catalytically, offer improved recyclability, lower raw material scarcity, and reduced environmental impact through corrosion resistance and longer lifecycle. Sustainable industrial catalysis increasingly favors steel alloys enhanced with minimal noble metal content to balance performance with reduced carbon emissions and resource depletion.
Industrial Applications: Platinum vs Steel
Platinum is widely favored in industrial catalysis due to its exceptional resistance to corrosion and superior catalytic activity, making it ideal for processes like automotive catalytic converters and chemical synthesis. Steel, particularly stainless steel, offers mechanical strength and cost-efficiency but generally lacks the catalytic efficiency of platinum, limiting its use to structural components rather than active catalytic sites. Industries prioritize platinum for reactions requiring high selectivity and durability under harsh conditions, while steel serves as a robust support material in catalytic systems.
Advances in Catalyst Technology
Advances in catalyst technology reveal platinum's superior activity and selectivity in industrial catalysis due to its unique electronic properties and resistance to sintering, enabling higher efficiency in reactions such as hydrogenation and reforming. Recent developments in steel-based catalysts focus on doping and nanostructuring to improve durability and cost-effectiveness, making steel a competitive alternative in processes where platinum's expense and scarcity limit large-scale application. Enhanced catalyst supports and alloy formulations continue to optimize the performance of both platinum and steel catalysts, driving innovation in energy conversion and chemical manufacturing industries.
Future Trends in Industrial Catalysis Materials
Platinum continues to dominate industrial catalysis due to its exceptional catalytic efficiency and resistance to deactivation, yet the high cost and limited availability drive research toward alternative materials like steel-based catalysts. Emerging trends highlight the development of steel alloys enhanced with nanostructured coatings or dopants to mimic platinum's active sites, aiming to balance performance with economic viability. Advances in atomic layer deposition and machine learning-assisted material design accelerate the discovery of steel-supported catalytic systems optimized for hydrogenation, oxidation, and reforming processes in sustainable industrial applications.
Infographic: Platinum vs Steel for Industrial Catalysis
 azmater.com
azmater.com