Osmium, one of the densest and most corrosion-resistant metals, offers superior stability and durability for advanced material research compared to francium, a highly radioactive and scarce alkali metal with limited practical applications. Research on osmium focuses on catalysis, electronics, and wear-resistant alloys, while francium's extreme instability restricts its use primarily to nuclear physics studies.
Table of Comparison
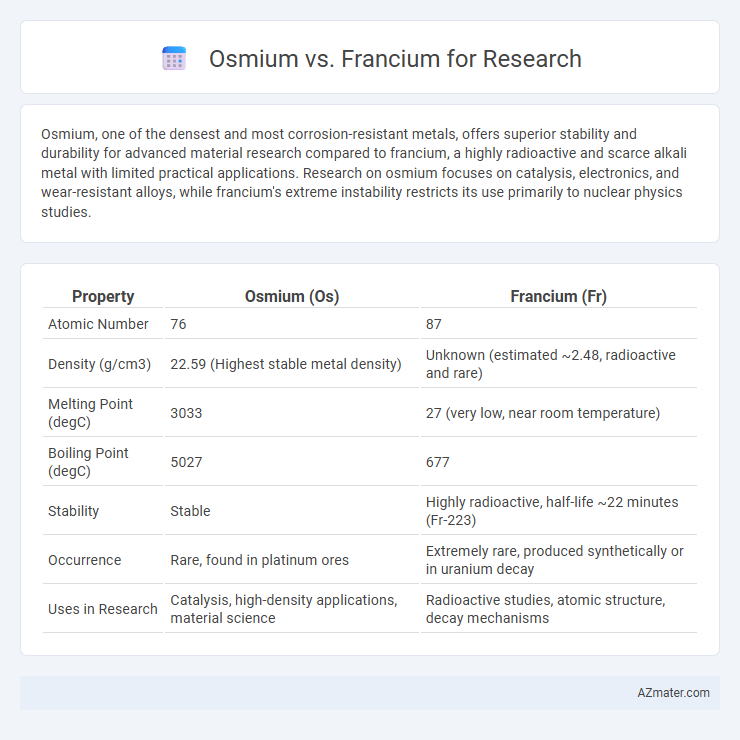
| Property | Osmium (Os) | Francium (Fr) |
|---|---|---|
| Atomic Number | 76 | 87 |
| Density (g/cm3) | 22.59 (Highest stable metal density) | Unknown (estimated ~2.48, radioactive and rare) |
| Melting Point (degC) | 3033 | 27 (very low, near room temperature) |
| Boiling Point (degC) | 5027 | 677 |
| Stability | Stable | Highly radioactive, half-life ~22 minutes (Fr-223) |
| Occurrence | Rare, found in platinum ores | Extremely rare, produced synthetically or in uranium decay |
| Uses in Research | Catalysis, high-density applications, material science | Radioactive studies, atomic structure, decay mechanisms |
Introduction to Osmium and Francium
Osmium is a dense, blue-gray transition metal known for its hardness, high melting point, and excellent corrosion resistance, making it valuable in scientific applications such as catalysis and material science research. Francium, a highly radioactive alkali metal with a very short half-life, is rarely found naturally and primarily studied for its properties in nuclear physics and atomic research. The stark differences in stability and availability between osmium and francium largely determine their distinct research applications and methodologies.
Elemental Properties Comparison
Osmium exhibits significant research interest due to its exceptional density of 22.59 g/cm3 and high melting point of 3045degC, making it ideal for studying extreme material properties and catalytic applications. Francium, with an atomic number of 87 and extreme radioactivity, presents challenges for research due to its scarcity and short half-life of approximately 22 minutes, limiting practical experimental studies. Comparing elemental properties, osmium's stability contrasts sharply with francium's instability, influencing their respective usability in chemical and physical research contexts.
Atomic Structure Differences
Osmium, with atomic number 76, has a dense, highly stable electron shell configuration [Xe] 4f14 5d6 6s2, contributing to its exceptional density and chemical inertness, making it suitable for precise material science studies. Francium, atomic number 87, exhibits a highly unstable, radioactive nature with an electron configuration of [Rn] 7s1, leading to a short half-life and limited availability, restricting extensive research applications. The stark contrast in their atomic structures, stability, and electron configurations profoundly affects their usability in research, with osmium favored for stable, long-term experiments and francium mainly studied in transient nuclear physics investigations.
Natural Occurrence and Abundance
Osmium, a dense transition metal, naturally occurs in the Earth's crust at approximately 0.0015 parts per million, making it one of the rarest stable elements commonly used in research involving catalysis and material science. In contrast, francium is a highly radioactive alkali metal with an extremely limited natural occurrence, found only in trace amounts--estimated at less than 30 grams in the entire Earth's crust--due to its short half-life and continuous radioactive decay. The significant disparity in natural abundance between osmium and francium impacts their availability and practical application in scientific studies, with osmium being more accessible for experimental use.
Synthesis and Isolation Methods
Osmium is synthesized primarily through the processing of platinum ores, where it is isolated by fractional crystallization and chemical precipitation due to its high density and low reactivity. Francium, an extremely rare and highly radioactive alkali metal, is produced synthetically via alpha decay of actinium or by bombarding thorium with protons, with isolation limited to trace quantities using ion-exchange chromatography techniques. The complexity of francium's synthesis and rapid decay pose significant challenges compared to osmium's more stable and abundant characteristics, impacting their respective research applications.
Chemical Reactivity and Stability
Osmium exhibits exceptional chemical stability with low reactivity, making it ideal for research requiring inertness and resistance to corrosion. Francium, as a highly radioactive alkali metal, displays extreme chemical reactivity but has a very short half-life, limiting its practical use in experimental studies. Researchers often choose osmium for stable, long-term chemical experiments, while francium's fleeting existence confines its applications to theoretical or nuclear chemistry investigations.
Safety and Handling Precautions
Osmium, a dense transition metal, poses minimal radioactive hazards but requires caution due to its toxic osmium tetroxide compound, mandating use of fume hoods and protective gloves during handling. Francium, an extremely rare and highly radioactive alkali metal, demands rigorous safety protocols including remote handling and extensive shielding to prevent radiation exposure. Research environments prioritize osmium for stability, whereas francium's intense radioactivity restricts its use to specialized, tightly controlled nuclear laboratories.
Applications in Scientific Research
Osmium's exceptional density and hardness make it ideal for applications in electron microscopy and as a catalyst in chemical reactions, enhancing material analysis and synthesis. Francium, highly radioactive and scarce, is primarily utilized in fundamental physics research, particularly in studies of atomic structure and weak interactions. The contrasting properties of osmium and francium drive their specialized roles in advancing scientific understanding across material science and nuclear physics.
Challenges in Experimental Use
Osmium's high density and toxicity pose significant challenges in sample handling and experimental setups, requiring specialized equipment and safety protocols. Francium's extreme radioactivity and short half-life make it difficult to produce and study, limiting experimental observations to transient states and demanding rapid, highly controlled conditions. Both elements require advanced containment and detection technologies, with francium experiments further constrained by limited availability and rapid decay.
Future Directions in Elemental Studies
Osmium's remarkable density and chemical stability make it a prime candidate for advanced material science research, including applications in catalysis and high-precision instrumentation. Francium, despite its extreme radioactivity and scarcity, offers unique opportunities in nuclear physics and atomic structure studies due to its position as the heaviest alkali metal. Future directions in elemental studies emphasize developing safer handling techniques for francium and exploring osmium's catalytic properties for sustainable technologies.
Infographic: Osmium vs Francium for Research
 azmater.com
azmater.com