Antimony enhances firework effects by producing sparkles and white flashes, while strontium provides vivid red colors due to its bright red flame emission. Antimony compounds increase spark intensity and duration, whereas strontium salts are key for vibrant red pyrotechnic displays.
Table of Comparison
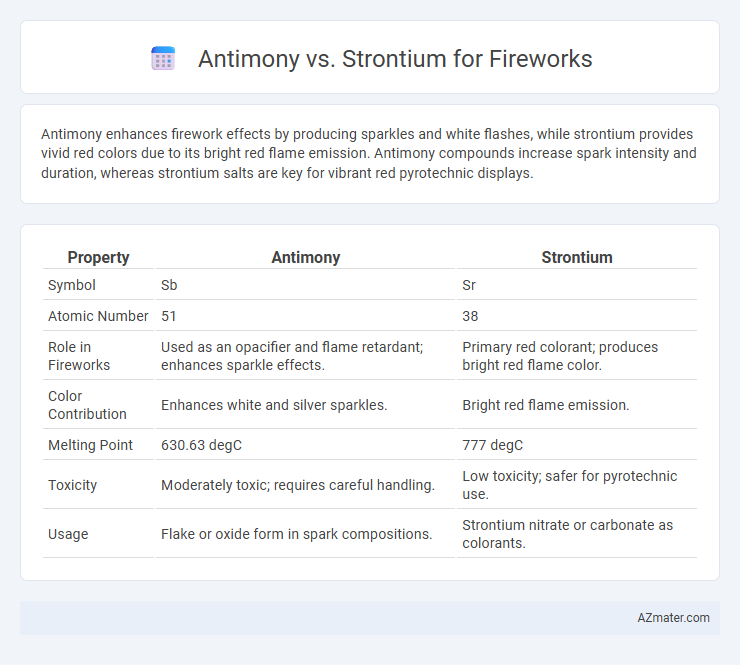
| Property | Antimony | Strontium |
|---|---|---|
| Symbol | Sb | Sr |
| Atomic Number | 51 | 38 |
| Role in Fireworks | Used as an opacifier and flame retardant; enhances sparkle effects. | Primary red colorant; produces bright red flame color. |
| Color Contribution | Enhances white and silver sparkles. | Bright red flame emission. |
| Melting Point | 630.63 degC | 777 degC |
| Toxicity | Moderately toxic; requires careful handling. | Low toxicity; safer for pyrotechnic use. |
| Usage | Flake or oxide form in spark compositions. | Strontium nitrate or carbonate as colorants. |
Introduction to Antimony and Strontium in Fireworks
Antimony is primarily used in fireworks as an oxidizer and a pigment, producing sparkling effects and enhancing flame colors due to its high thermal stability and ability to combine with other metals. Strontium compounds, especially strontium carbonate and strontium nitrate, are essential in fireworks for generating vibrant red flames and improving the color purity of pyrotechnic displays. Both elements contribute distinct roles; antimony adds texture and brightness, while strontium focuses on color intensity and flame quality, making them critical in formulating various firework effects.
Chemical Properties and Roles in Pyrotechnics
Antimony serves as a common oxidizer and a flame color modifier in fireworks due to its ability to enhance the brilliance and intensity of colors, particularly gold and silver hues. Strontium compounds, especially strontium nitrate and strontium carbonate, are crucial for producing vibrant red flames, attributed to the strong emission lines in the red spectral region when burned. The contrasting chemical properties--antimony's capacity to catalyze combustion reactions and strontium's efficient light emission at specific wavelengths--define their complementary roles in pyrotechnic formulations.
Color Effects: Antimony vs Strontium
Strontium produces vibrant red hues in fireworks due to its ability to emit bright red light when heated, making it a preferred element for red color effects in pyrotechnics. Antimony, on the other hand, is primarily used as a flame retardant and to enhance the luminosity and sparkle rather than imparting strong color, contributing more to the white sparks and brilliance in firework displays. The combination of strontium for vivid red and antimony for spark effects creates dynamic and visually striking fireworks compositions.
Burn Characteristics and Performance
Antimony enhances firework effects by producing dense, white smoke and helping to stabilize the composition through its burn rate control properties. Strontium is primarily valued for its vivid red flame and contributes to cleaner, more intense combustion with less smoke output. Combining antimony and strontium can optimize burn characteristics, balancing opacity and brightness for superior visual performance in fireworks.
Safety Considerations and Handling
Antimony compounds in fireworks enhance flame color but pose significant toxicity risks, requiring strict handling protocols with protective gear and proper ventilation. Strontium salts provide vivid red hues while exhibiting lower toxicity, making them safer alternatives for pyrotechnic applications. Both elements demand careful storage away from moisture and incompatible materials to prevent hazardous reactions and ensure operator safety.
Environmental Impact of Antimony and Strontium
Antimony used in fireworks poses significant environmental risks due to its toxicity and persistence in soil and water, leading to bioaccumulation in wildlife and potential human health hazards. Strontium, while also a heavy metal, generally exhibits lower toxicity and environmental persistence, making it a somewhat safer alternative for color effects in pyrotechnics. However, both metals require careful management to minimize environmental contamination from fireworks residue.
Cost and Availability for Firework Production
Antimony is commonly used in fireworks for its flame color and spark effects but tends to be more expensive and less abundant compared to strontium. Strontium, primarily used for producing vibrant red colors in pyrotechnics, offers a more cost-effective and readily available option due to its widespread mining and lower market price. Firework manufacturers often prefer strontium over antimony to balance vivid color production with budget constraints and supply chain reliability.
Historical Uses in Pyrotechnic Displays
Antimony compounds have historically been used as flame retardants and stabilizers in pyrotechnic mixtures, contributing to dense white smoke and enhancing explosion effects in fireworks. Strontium salts, notably strontium nitrate and strontium carbonate, have been extensively employed for their vivid red coloration in firework displays since the 19th century. The choice between antimony and strontium in pyrotechnics reflects a balance between visual impact and combustion behavior, with strontium dominating color production and antimony influencing smoke and stability.
Innovations and Alternatives in Firework Chemistry
Antimony compounds have traditionally enhanced spark brightness and stability in fireworks, but their toxicity has driven the search for safer alternatives like strontium, which produces vivid red hues with lower environmental impact. Innovations in firework chemistry now emphasize strontium-based formulations to replace antimony sulfides, improving color purity while reducing harmful emissions. Researchers continue to explore strontium complexes and nanomaterials to optimize ignition properties and brightness, advancing eco-friendly pyrotechnic compositions.
Choosing the Right Element: Antimony or Strontium
Antimony and strontium serve distinct purposes in fireworks, with antimony primarily used as a flame retardant and strontium as a vibrant red colorant. Selecting strontium enhances red hues due to its ability to emit bright red light when burned, while antimony improves the stability and safety of the pyrotechnic composition. For vivid coloration in fireworks, strontium is the preferred choice, whereas antimony is chosen to reduce flammability and control burn rate.
Infographic: Antimony vs Strontium for Firework
 azmater.com
azmater.com