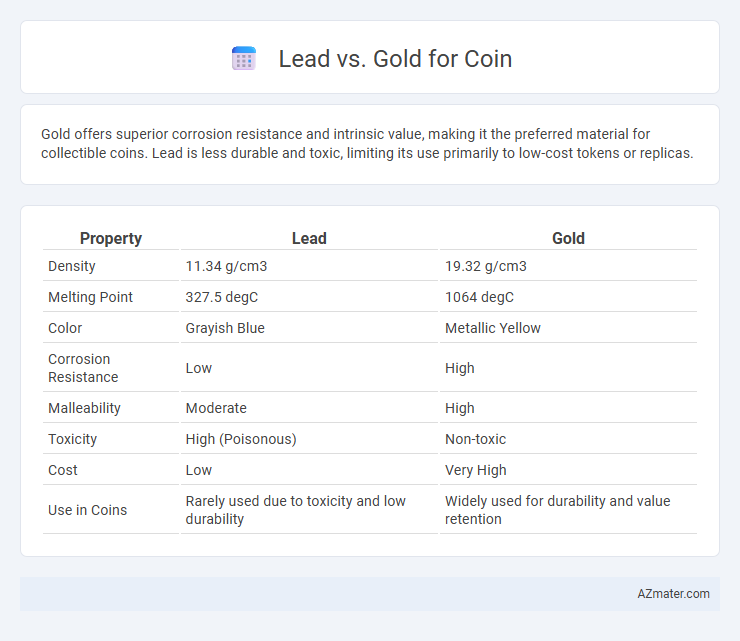
Gold offers superior corrosion resistance and intrinsic value, making it the preferred material for collectible coins. Lead is less durable and toxic, limiting its use primarily to low-cost tokens or replicas.
Table of Comparison
| Property | Lead | Gold |
|---|---|---|
| Density | 11.34 g/cm3 | 19.32 g/cm3 |
| Melting Point | 327.5 degC | 1064 degC |
| Color | Grayish Blue | Metallic Yellow |
| Corrosion Resistance | Low | High |
| Malleability | Moderate | High |
| Toxicity | High (Poisonous) | Non-toxic |
| Cost | Low | Very High |
| Use in Coins | Rarely used due to toxicity and low durability | Widely used for durability and value retention |
Introduction to Lead and Gold in Coinage
Lead and gold have historically played distinct roles in coinage due to their differing physical properties and economic values. Gold, prized for its rarity and durability, has been the standard for high-value coins and wealth storage since ancient civilizations. Lead, being abundant and malleable but less valuable and more prone to corrosion, was typically used for low-value or token coinage rather than official currency.
Historical Use of Lead and Gold Coins
Lead coins were historically valued for their low cost and ease of production, often used for small denominations or in regions facing metal shortages, despite their susceptibility to corrosion and lack of intrinsic value. Gold coins, widely recognized since ancient civilizations like the Lydians and Romans, symbolized wealth and stability due to gold's rarity, resistance to tarnish, and high economic value. The contrast in these metals' properties influenced their coinage use: lead served practical, limited purposes, while gold coins represented trust and permanence in monetary systems.
Chemical and Physical Properties Comparison
Lead exhibits a density of 11.34 g/cm3 and a melting point of 327.5degC, while gold features a higher density at 19.32 g/cm3 and a melting point of 1064degC, reflecting greater mass and thermal stability. Chemically, lead is a post-transition metal with a tendency to oxidize and form lead oxides, whereas gold is a noble metal renowned for its excellent corrosion resistance and inertness. Physically, gold is highly malleable and ductile compared to lead, which is softer but prone to tarnishing and oxidation under environmental exposure.
Rarity and Value: Lead vs. Gold
Gold coins are highly valued and rare due to gold's intrinsic worth, scarcity, and historical role as a currency standard, making them desirable for collectors and investors. Lead coins, often produced as inexpensive or counterfeit alternatives, lack intrinsic value and rarity, resulting in minimal market demand and low collectible worth. The significant contrast in rarity and value between gold and lead coins underscores gold's prominence in numismatics and investment markets.
Durability and Longevity of Lead and Gold Coins
Gold coins exhibit superior durability and longevity compared to lead coins due to gold's resistance to corrosion, tarnish, and wear over time. Lead coins are prone to oxidation and physical degradation, resulting in diminished clarity and structural integrity. The inherent stability of gold makes it a preferred choice for minting coins intended to endure for centuries.
Economic Impacts of Using Lead or Gold
Using lead for coins significantly reduces production costs due to its low market price, but it increases economic risks associated with health hazards and environmental cleanup expenses. Gold coins, though costly to produce, provide greater long-term economic value through durability, intrinsic precious metal worth, and higher public trust, supporting stable monetary systems. The choice between lead and gold directly affects currency longevity, recycling potential, and overall financial stability in an economy.
Counterfeiting Concerns: Lead and Gold Coins
Lead coins pose significant counterfeiting risks due to their low density and malleability, making them easier to replicate but simpler to detect by weight and magnetism. Gold coins, with their high density, distinct color, and unique metallic properties, are much harder to counterfeit, offering greater authenticity assurance in numismatics. Advanced techniques like X-ray fluorescence and ultrasonic testing further differentiate genuine gold coins from lead replicas, enhancing security against forgery.
Environmental and Health Effects
Lead coins pose significant environmental and health risks due to their toxicity, which can cause soil and water contamination and lead to neurological damage upon prolonged exposure. Gold coins, being inert and non-toxic, present minimal environmental hazards and are safer for both human handling and ecological systems. The preference for gold in coinage reduces the risk of heavy metal poisoning and contamination, making it a more sustainable and health-conscious choice.
Modern Applications in Numismatics
Lead coins, once common as low-cost alternatives, exhibit poor durability and are prone to corrosion, limiting their use in modern numismatics where preservation and authenticity are crucial. Gold coins remain the standard in contemporary numismatic applications due to their intrinsic value, resistance to tarnish, and appeal in investment-grade collections. Advances in metallurgical analysis and digital authentication tools enhance the ability of collectors and institutions to distinguish genuine gold coins from lead counterfeits, maintaining gold's dominance in the field.
Conclusion: Which Metal is Superior for Coins?
Gold is the superior metal for coins due to its rarity, intrinsic value, and resistance to corrosion, making it a longstanding symbol of wealth and stability. Lead, being soft, toxic, and prone to oxidation, lacks durability and economic value, rendering it unsuitable for coinage. The inherent properties of gold ensure longevity, aesthetic appeal, and trust in monetary systems worldwide.
Infographic: Lead vs Gold for Coin
 azmater.com
azmater.com