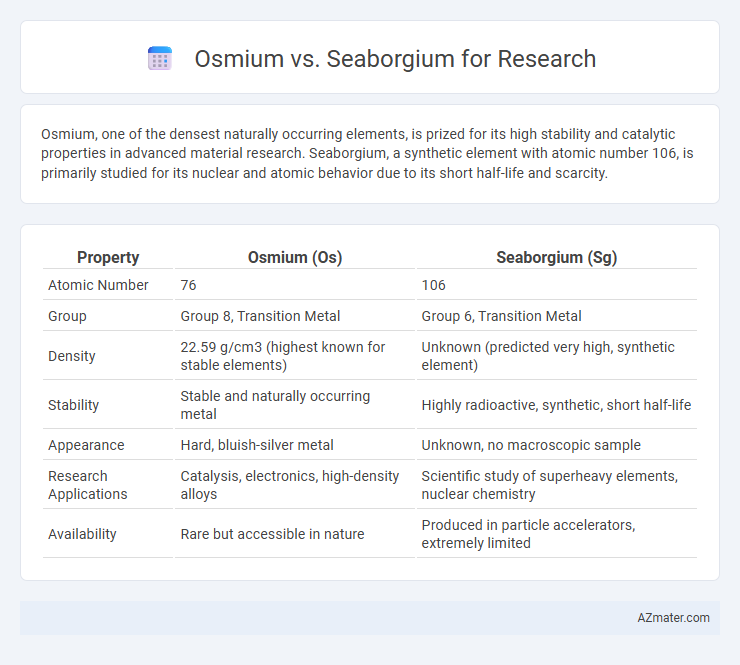
Osmium, one of the densest naturally occurring elements, is prized for its high stability and catalytic properties in advanced material research. Seaborgium, a synthetic element with atomic number 106, is primarily studied for its nuclear and atomic behavior due to its short half-life and scarcity.
Table of Comparison
| Property | Osmium (Os) | Seaborgium (Sg) |
|---|---|---|
| Atomic Number | 76 | 106 |
| Group | Group 8, Transition Metal | Group 6, Transition Metal |
| Density | 22.59 g/cm3 (highest known for stable elements) | Unknown (predicted very high, synthetic element) |
| Stability | Stable and naturally occurring metal | Highly radioactive, synthetic, short half-life |
| Appearance | Hard, bluish-silver metal | Unknown, no macroscopic sample |
| Research Applications | Catalysis, electronics, high-density alloys | Scientific study of superheavy elements, nuclear chemistry |
| Availability | Rare but accessible in nature | Produced in particle accelerators, extremely limited |
Introduction to Osmium and Seaborgium
Osmium, a dense transition metal with atomic number 76, is highly valued in research for its exceptional hardness, high melting point, and unique catalytic properties, making it pivotal in fields like material science and chemistry. Seaborgium, a synthetic element with atomic number 106, is primarily studied in nuclear chemistry and physics due to its short half-life and transuranium characteristics, providing insights into atomic structure and heavy element synthesis. Research comparing Osmium and Seaborgium highlights the contrast between naturally occurring elements used in practical applications and synthetic elements crucial for expanding the periodic table and understanding nuclear stability.
Chemical and Physical Properties Comparison
Osmium exhibits exceptional density (22.59 g/cm3) and high melting point (3045 K), making it ideal for applications requiring extreme durability and stability in chemical research. Seaborgium, a synthetic transactinide element with a short half-life (~14 seconds for isotope Sg-269), is primarily studied for its nuclear properties rather than chemical behavior, limiting its practical use in physical experiments. Comparative analysis highlights osmium's well-characterized electron configuration ([Xe] 4f14 5d6 6s2) contrasting with seaborgium's predicted, but experimentally challenging, chemistry due to rapid radioactive decay and scarcity.
Natural Occurrence and Synthesis
Osmium, a naturally occurring element in the platinum group metals, is found in Earth's crust with an average abundance of approximately 1 part per billion, making it accessible for research through mining and refining processes. In contrast, seaborgium is a synthetic element with no stable isotopes and is produced exclusively in particle accelerators via nuclear reactions, limiting its availability to minute, short-lived quantities. The natural occurrence of osmium facilitates extensive experimental studies, while seaborgium's synthesis constraints restrict research primarily to theoretical and short-term experimental investigations.
Applications in Scientific Research
Osmium's dense atomic structure makes it ideal for high-precision instruments and catalysis in chemical research, especially in oxidation reactions and hardness studies. Seaborgium, a synthetic transactinide element with a very short half-life, is primarily used in nuclear physics experiments to explore the properties of superheavy elements and test theories of nuclear stability. Due to its rarity and radioactivity, seaborgium's applications are limited to fundamental scientific research rather than practical technological uses.
Challenges in Handling and Storage
Osmium, a dense and volatile metal, poses significant challenges in handling due to its toxic osmium tetroxide fumes that require stringent ventilation and protective equipment. Seaborgium, a synthetic element with no stable isotopes and a half-life measured in seconds, presents extreme difficulties for storage and experimentation because it rapidly decays and can only be produced in particle accelerators in minute quantities. Both elements demand specialized containment protocols, but seaborgium's fleeting existence limits practical research applications compared to the hazardous but more stable osmium.
Toxicity and Safety Considerations
Osmium poses significant toxicity risks due to osmium tetroxide, a highly volatile and toxic compound that demands strict handling protocols and protective equipment in research environments. Seaborgium, a synthetic element with no stable isotopes, is produced in minute quantities with extreme radioactivity, requiring advanced containment and remote handling systems to ensure researcher safety. Safety considerations prioritize minimizing exposure to osmium's chemical hazards and managing the radiological risks associated with seaborgium's short half-life and intense radioactivity.
Cost and Availability for Laboratories
Osmium is a naturally occurring element with significant availability and a relatively high cost due to its rarity and density, making it accessible albeit expensive for research laboratories. Seaborgium, being a synthetic element produced only in particle accelerators with extremely limited quantities, is prohibitively expensive and virtually unavailable for most research purposes. Laboratories prioritizing cost-effective and accessible materials typically favor osmium over seaborgium for experimental and analytical applications.
Recent Research Advances
Osmium has been extensively studied for its catalytic properties and high-density applications, with recent research highlighting its role in nanomaterials and electrochemical sensors. Seaborgium, a synthetic transactinide element, remains challenging to investigate due to its short half-life, but recent advances in rapid synthesis and detection techniques have enabled limited exploration of its chemical behavior and isotope stability. Comparative studies emphasize osmium's established utility in practical applications while seaborgium's research primarily expands fundamental understanding of superheavy element properties.
Suitability for Experimental Studies
Osmium's high density and chemical stability make it ideal for experimental studies requiring durable and inert heavy metals, especially in catalysis and material science research. Seaborgium, a synthetic element with a very short half-life and limited availability, poses significant challenges for experimental studies due to its rapid decay and difficulty in producing sufficient quantities. Consequently, osmium is far more suitable for detailed and reproducible experimental research compared to seaborgium.
Future Prospects and Innovations
Osmium, with its extreme density and stability, remains a crucial element for advancements in catalysis and high-precision instruments, promising innovations in materials science and electronics. Seaborgium, a synthetic and highly radioactive element, offers limited practical applications but holds significant potential for nuclear chemistry and particle physics research, advancing our understanding of superheavy elements. Future prospects center on enhancing osmium's applications in nanotechnology while exploring seaborgium's role in expanding the periodic table and nuclear synthesis techniques.
Infographic: Osmium vs Seaborgium for Research
 azmater.com
azmater.com