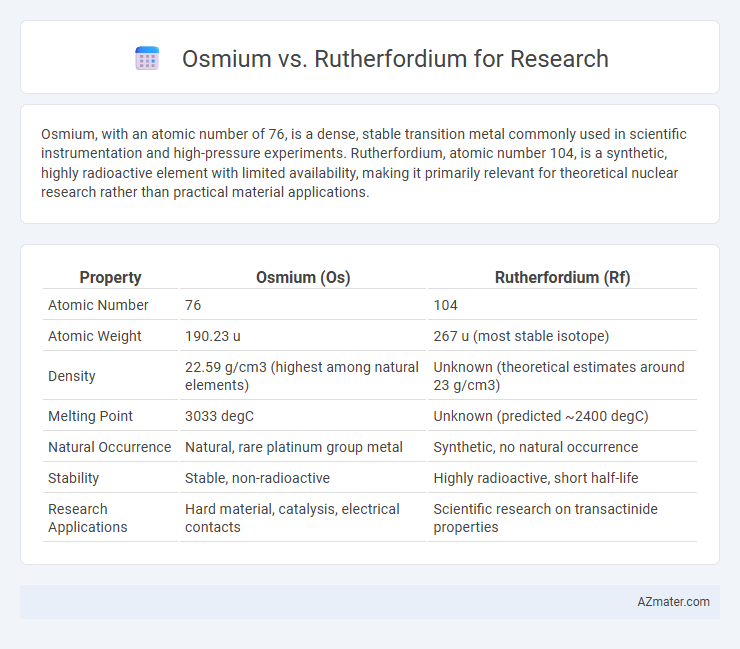
Osmium, with an atomic number of 76, is a dense, stable transition metal commonly used in scientific instrumentation and high-pressure experiments. Rutherfordium, atomic number 104, is a synthetic, highly radioactive element with limited availability, making it primarily relevant for theoretical nuclear research rather than practical material applications.
Table of Comparison
| Property | Osmium (Os) | Rutherfordium (Rf) |
|---|---|---|
| Atomic Number | 76 | 104 |
| Atomic Weight | 190.23 u | 267 u (most stable isotope) |
| Density | 22.59 g/cm3 (highest among natural elements) | Unknown (theoretical estimates around 23 g/cm3) |
| Melting Point | 3033 degC | Unknown (predicted ~2400 degC) |
| Natural Occurrence | Natural, rare platinum group metal | Synthetic, no natural occurrence |
| Stability | Stable, non-radioactive | Highly radioactive, short half-life |
| Research Applications | Hard material, catalysis, electrical contacts | Scientific research on transactinide properties |
Introduction to Osmium and Rutherfordium
Osmium, a dense transition metal in the platinum group, exhibits exceptional hardness and high melting point, making it valuable for applications in catalysis, electrical contacts, and fountain pen tips. Rutherfordium, a synthetic transactinide element with atomic number 104, is primarily studied in nuclear chemistry and physics due to its short half-life and radioactive properties. Research comparing osmium and rutherfordium focuses on osmium's stable chemical behavior versus rutherfordium's limited availability and radioactive nature, challenging detailed practical investigations.
Atomic Structure and Properties
Osmium, with atomic number 76, is a dense transition metal known for its high atomic density and complex electron configuration [Xe] 4f^14 5d^6 6s^2, making it valuable for studying heavy metal electron interactions and catalytic properties. Rutherfordium, atomic number 104, is a synthetic transactinide element with a short half-life and limited experimental data, featuring predicted electron configuration [Rn] 5f^14 6d^2 7s^2 that challenges understanding of relativistic effects on atomic structure in superheavy elements. Research comparing osmium's stable electronic and crystalline properties to rutherfordium's theoretical models enhances insight into periodic trends and quantum behaviors in high-Z elements.
Natural Occurrence and Synthesis
Osmium, a naturally occurring element found in platinum ores, is one of the densest metals and is extensively used in research due to its availability and stability. Rutherfordium, a synthetic element with atomic number 104, does not occur naturally and must be produced in particle accelerators through nuclear reactions involving lighter elements like californium or plutonium. The limited synthesis and short half-life of rutherfordium restrict its practical research applications compared to naturally abundant osmium.
Stability and Isotopic Variations
Osmium offers remarkable stability with its most abundant isotope, Os-192, providing consistent performance in research applications, while Rutherfordium, being a synthetic element with no stable isotopes, exhibits rapid radioactive decay limiting its practical use. Osmium's isotopic variations, including seven naturally occurring isotopes, allow researchers to study isotopic effects and nuclear properties extensively. Rutherfordium's isotopes, primarily produced in particle accelerators, are highly unstable with half-lives measured in seconds, restricting investigations to short-lived nuclear reactions and synthesis studies.
Physical and Chemical Characteristics
Osmium exhibits a high density of approximately 22.59 g/cm3 and exceptional hardness, making it one of the densest naturally occurring elements with a melting point of 3045degC, while Rutherfordium, a synthetic transactinide element, has an estimated melting point near 2400degC but remains largely uncharacterized due to its short half-life and limited availability. Chemically, Osmium is highly resistant to corrosion and oxidation, commonly forming OsO4, a volatile and highly toxic compound, whereas Rutherfordium demonstrates expected group 4 behavior similar to hafnium and zirconium, typically forming +4 oxidation states in aqueous solutions. Research applications prioritize Osmium for its stability and unique catalytic properties in organic synthesis, whereas Rutherfordium's investigation focuses on fundamental nuclear properties and relativistic effects impacting its electron configuration and chemical bonding.
Research Applications and Uses
Osmium, a dense transition metal, is primarily utilized in high-precision instruments, catalysis, and as a standard for hardness testing due to its durability and chemical stability. Rutherfordium, a synthetic and highly radioactive element, has limited research applications confined mostly to nuclear chemistry and physics experiments aimed at understanding superheavy elements and their properties. Osmium's practical uses contrast sharply with Rutherfordium's theoretical significance in advancing knowledge of the periodic table's transactinide region.
Experimental Challenges and Handling
Osmium's extreme density and toxicity pose significant experimental challenges in laboratory handling, requiring specialized containment and ventilation systems to minimize exposure risks. Rutherfordium, a synthetic element with a very short half-life and rare production, demands rapid and highly sensitive detection techniques in particle accelerators for its study. The handling of Rutherfordium involves intricate coordination between nuclear facilities and advanced instrumentation due to its radioactivity and scarcity, contrasting with osmium's more stable but chemically hazardous nature.
Availability and Cost Considerations
Osmium, a rare dense metal with a global annual production of around 100 kilograms, is more accessible and affordable for research compared to Rutherfordium, a synthetic element produced only in milligram quantities through nuclear reactions. The high cost of Rutherfordium stems from its complex synthesis in particle accelerators, making it impractical for extensive experimental use outside specialized nuclear research facilities. Osmium's relative availability and lower price enable broader applications in catalysis, electronics, and material science research.
Safety and Environmental Impact
Osmium, a dense and stable transition metal, poses significant safety risks due to its toxic osmium tetroxide compound, which is highly volatile and harmful upon inhalation or skin contact, necessitating stringent handling protocols in research environments. Rutherfordium, a synthetic, highly radioactive transactinide element with a short half-life, presents extreme radiological hazards that require specialized containment and disposal procedures to protect researchers and minimize environmental contamination. The environmental impact of osmium involves potential toxicity and bioaccumulation, while rutherfordium's radioactive decay demands rigorous waste management to prevent long-term ecological damage.
Future Prospects in Scientific Research
Osmium, a dense transition metal with stable isotopes, continues to offer valuable applications in catalysis, materials science, and electronic compounds due to its well-characterized properties and availability. Rutherfordium, a synthetic element with no stable isotopes and a very short half-life, presents significant challenges for practical research but holds potential for advancing understanding of superheavy element chemistry and nuclear physics. Future prospects favor osmium in applied research fields, while rutherfordium remains primarily a subject of fundamental scientific investigation in laboratories equipped for handling highly radioactive materials.
Infographic: Osmium vs Rutherfordium for Research
 azmater.com
azmater.com