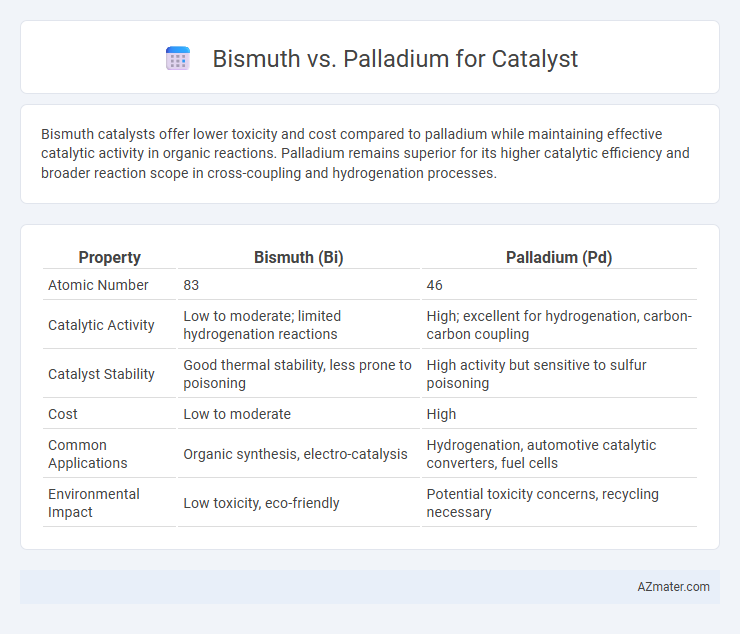
Bismuth catalysts offer lower toxicity and cost compared to palladium while maintaining effective catalytic activity in organic reactions. Palladium remains superior for its higher catalytic efficiency and broader reaction scope in cross-coupling and hydrogenation processes.
Table of Comparison
| Property | Bismuth (Bi) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 83 | 46 |
| Catalytic Activity | Low to moderate; limited hydrogenation reactions | High; excellent for hydrogenation, carbon-carbon coupling |
| Catalyst Stability | Good thermal stability, less prone to poisoning | High activity but sensitive to sulfur poisoning |
| Cost | Low to moderate | High |
| Common Applications | Organic synthesis, electro-catalysis | Hydrogenation, automotive catalytic converters, fuel cells |
| Environmental Impact | Low toxicity, eco-friendly | Potential toxicity concerns, recycling necessary |
Introduction to Bismuth and Palladium as Catalysts
Bismuth and palladium are prominent catalysts in organic synthesis and industrial applications, each exhibiting unique properties that influence reaction outcomes. Bismuth, known for its low toxicity and environmental friendliness, serves as an effective catalyst in oxidation and coupling reactions, particularly in green chemistry. Palladium, a noble metal with exceptional catalytic activity, is widely employed in cross-coupling reactions, hydrogenation, and carbon-carbon bond formation, making it indispensable in pharmaceutical and fine chemical manufacturing.
Chemical Properties and Catalytic Mechanisms
Bismuth exhibits low toxicity and unique electronic configuration influencing its catalytic activity, often promoting selective oxidation reactions through facile electron transfer and stable surface oxides. Palladium features exceptional hydrogen absorption and activation capabilities, facilitating catalytic cycles in hydrogenation and carbon-carbon coupling via its d-orbital electron density and variable oxidation states. The catalytic mechanisms differ as bismuth catalysts rely on altering electronic environments to favor reactant adsorption and selective pathways, while palladium catalysis involves dynamic formation of metal-hydride intermediates crucial for bond activation and product formation.
Historical Use in Catalysis
Bismuth and palladium have distinct historical roles in catalysis, with palladium extensively employed since the mid-20th century for cross-coupling reactions and hydrogenation processes due to its exceptional catalytic activity and selectivity. Bismuth, traditionally less common, gained interest in recent decades as a non-toxic, environmentally friendly alternative, especially in organic synthesis and oxidation reactions. The contrasting toxicological profiles and catalytic efficiencies have shaped their respective applications, with palladium dominating industrial catalysis and bismuth emerging in niche, eco-conscious catalytic processes.
Efficiency Comparison in Key Reactions
Bismuth catalysts exhibit superior selectivity and lower toxicity in selective oxidation and hydrogenation reactions compared to palladium, which is highly efficient but prone to deactivation and higher cost. In carbon-carbon coupling reactions such as Suzuki and Heck, palladium demonstrates significantly higher turnover frequencies and broader substrate tolerance, making it more efficient for complex synthetic processes. Efficiency in catalytic performance thus depends on the reaction type, with bismuth favored for environmentally benign transformations and palladium preferred for industrial-scale organic synthesis.
Environmental Impact and Toxicity
Bismuth exhibits low toxicity and is considered an environmentally friendly catalyst due to its minimal bioaccumulation and non-toxic nature in aquatic systems. Palladium, while highly effective as a catalyst, poses concerns regarding potential toxicity and environmental persistence, raising issues related to heavy metal contamination. The use of bismuth catalysts supports sustainable chemistry by reducing ecological risks associated with metal leaching and hazardous waste.
Cost and Resource Availability
Bismuth offers a significant cost advantage over palladium as a catalyst due to its abundance and lower market price, which is approximately $14 per kilogram compared to palladium's $65,000 per kilogram. The abundant terrestrial availability of bismuth, primarily as a byproduct of lead, copper, and tin mining, ensures a stable supply chain, whereas palladium is a rare platinum-group metal with limited global reserves concentrated in Russia and South Africa. These factors make bismuth a more economically viable and sustainable choice for large-scale catalytic applications.
Stability and Catalyst Lifetime
Bismuth-based catalysts exhibit enhanced stability under harsh reaction conditions due to their resistance to sintering and poisoning, contributing to prolonged catalyst lifetime in various chemical processes. Palladium catalysts, while highly active, often suffer from deactivation caused by carbon deposition and metal agglomeration, which reduces their effective lifespan. Advanced bismuth alloy formulations and support materials further improve long-term stability, making them favorable for applications requiring durable catalytic performance.
Industrial Applications and Case Studies
Bismuth catalysts offer exceptional selectivity and environmental benefits in industrial processes such as organic synthesis and wastewater treatment, as demonstrated in case studies involving pharmaceutical manufacturing and heavy metal remediation. Palladium catalysts are widely utilized for their superior activity and efficiency in hydrogenation, carbon-carbon coupling, and automotive catalytic converters, with extensive industrial applications in petrochemical refining and fine chemical production. Comparative analyses highlight Bismuth's lower toxicity and cost advantages, while Palladium maintains dominance due to its superior catalytic performance and versatility in high-demand industrial settings.
Recent Advances in Catalyst Design
Recent advances in catalyst design show palladium remains dominant for high-efficiency hydrogenation and carbon-carbon coupling reactions due to its exceptional catalytic activity and selectivity. Bismuth, explored as a cost-effective and environmentally benign alternative, exhibits unique electronic properties that enhance catalytic stability and resistance to poisoning in oxidation and reduction processes. Emerging studies demonstrate bismuth-palladium bimetallic catalysts achieving synergistic effects, improving reaction rates and recyclability in sustainable chemical synthesis.
Future Perspectives in Green Chemistry
Bismuth catalysts, known for their low toxicity and environmental compatibility, offer promising future perspectives in green chemistry by enabling sustainable catalytic processes with reduced hazardous waste. Palladium remains a highly efficient catalyst for a wide range of organic transformations, but concerns over cost, scarcity, and toxicity drive the search for greener alternatives like bismuth. Advancements in bismuth-based catalytic systems could revolutionize sustainable synthesis by providing cost-effective, eco-friendly alternatives to palladium, aligning with global efforts to minimize environmental impact.
Infographic: Bismuth vs Palladium for Catalyst
 azmater.com
azmater.com