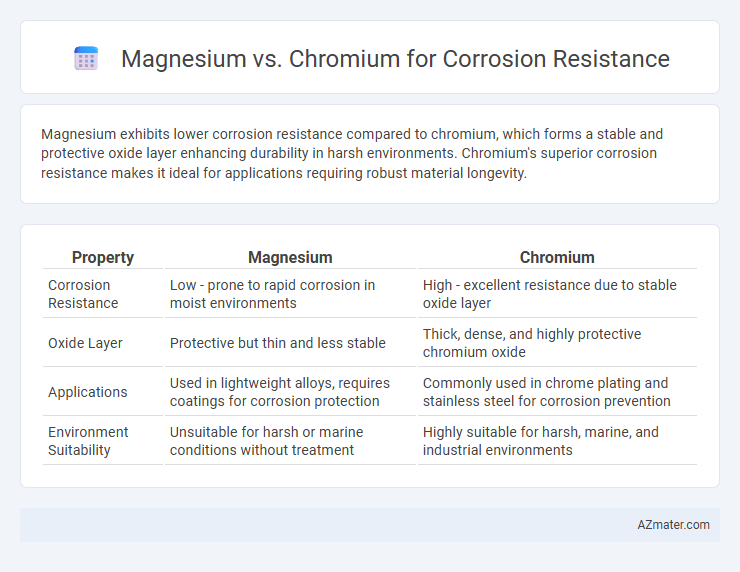
Magnesium exhibits lower corrosion resistance compared to chromium, which forms a stable and protective oxide layer enhancing durability in harsh environments. Chromium's superior corrosion resistance makes it ideal for applications requiring robust material longevity.
Table of Comparison
| Property | Magnesium | Chromium |
|---|---|---|
| Corrosion Resistance | Low - prone to rapid corrosion in moist environments | High - excellent resistance due to stable oxide layer |
| Oxide Layer | Protective but thin and less stable | Thick, dense, and highly protective chromium oxide |
| Applications | Used in lightweight alloys, requires coatings for corrosion protection | Commonly used in chrome plating and stainless steel for corrosion prevention |
| Environment Suitability | Unsuitable for harsh or marine conditions without treatment | Highly suitable for harsh, marine, and industrial environments |
Introduction to Corrosion Resistance
Magnesium and chromium exhibit distinct properties that influence their corrosion resistance in various environments. Magnesium, a lightweight metal, forms a thin oxide layer that offers limited protection against corrosion, especially in chloride-rich conditions. Chromium, known for its excellent corrosion resistance, enhances stainless steel by forming a stable, passive oxide film that significantly prevents rust and degradation.
Overview of Magnesium and Chromium
Magnesium is a lightweight metal known for its excellent corrosion resistance when alloyed or coated, often used in automotive and aerospace industries to reduce weight while maintaining durability. Chromium, a hard and corrosion-resistant metal, is widely utilized in stainless steel production and surface coatings due to its ability to form a passive oxide layer that prevents rust and degradation. Both elements enhance corrosion resistance but serve different industrial purposes: magnesium excels in structural lightweight applications while chromium is preferred for protective coatings and alloy stabilization.
Fundamental Properties Affecting Corrosion
Magnesium exhibits a lower standard electrode potential (-2.37 V) compared to chromium (-0.74 V), making magnesium more prone to oxidation and faster corrosion in most environments. Chromium's ability to form a stable, adherent chromium oxide (Cr2O3) passive layer significantly enhances its corrosion resistance by preventing further metal degradation. The fundamental difference lies in chromium's corrosion-resisting passive film versus magnesium's higher electrochemical activity and susceptibility to galvanic corrosion.
Mechanisms of Corrosion: Magnesium vs Chromium
Magnesium resists corrosion primarily through the formation of a thin, protective magnesium hydroxide layer that can deteriorate in chloride-rich environments, leading to localized pitting. Chromium enhances corrosion resistance by forming a stable, adherent chromium oxide passive film that effectively prevents further oxidation and protects underlying metal surfaces. The chromium oxide barrier is more chemically stable and insoluble compared to magnesium's protective layer, making chromium alloys superior in corrosive and aggressive environments.
Applications Requiring Corrosion Resistance
Magnesium alloys are widely used in automotive and aerospace industries due to their lightweight properties but generally offer lower corrosion resistance compared to chromium-enhanced steel or coatings. Chromium provides superior corrosion resistance in applications requiring durable protective layers, such as in industrial machinery, automotive body parts, and marine equipment, by forming a stable, passive oxide film. For environments with high moisture or chemical exposure, chromium plating or stainless steel with chromium content is preferred over magnesium alloys to ensure long-term corrosion protection.
Comparative Corrosion Performance
Magnesium exhibits rapid corrosion in aqueous environments due to its highly active electrochemical potential, whereas chromium forms a passive oxide layer that significantly enhances corrosion resistance, especially in stainless steel alloys. Chromium's ability to create a protective, adherent chromium oxide film limits metal ion release and protects substrates from pitting and crevice corrosion in chloride-rich settings. Comparative studies demonstrate that chromium-enriched coatings or alloys outperform magnesium in durability and longevity under corrosive conditions, making chromium ideal for applications demanding high corrosion resistance.
Protective Coatings: Impact on Both Metals
Magnesium coatings offer excellent corrosion resistance through the formation of a stable oxide layer that protects the underlying metal, often used as sacrificial anodes in galvanic protection systems. Chromium-based coatings, such as chromium plating, create a hard, dense barrier that resists oxidation and wear, significantly extending the metal's lifespan in harsh environments. Both metals benefit from protective coatings, but while magnesium relies on active corrosion protection, chromium provides a primarily passive, physical shield against corrosion.
Environmental Factors Influencing Corrosion
Magnesium exhibits high susceptibility to corrosion in environments with high humidity and chloride presence, while chromium forms a passive oxide layer that enhances its resistance to such conditions. Environmental factors like acidic rain, saline water, and temperature fluctuations accelerate the corrosion of magnesium alloys, whereas chromium-based coatings maintain stability and durability under these stressors. The electrochemical properties of chromium significantly reduce oxidation rates, making it a superior choice for corrosion resistance in harsh environmental settings.
Cost and Availability Considerations
Magnesium offers a balance of lightweight properties and moderate corrosion resistance but tends to be more expensive and less abundant than chromium, impacting cost-efficiency in large-scale applications. Chromium provides superior corrosion resistance at a lower cost due to its higher availability and widespread use in coatings and alloys, making it more economically viable for industrial purposes. Cost-effectiveness and material availability heavily favor chromium when long-term corrosion protection is critical and budget constraints are considered.
Conclusion: Choosing the Right Metal for Corrosion Resistance
Magnesium offers lightweight advantages but has lower corrosion resistance compared to chromium, which provides superior protection due to its ability to form stable oxide layers. Chromium's resistance to oxidation and tarnishing makes it ideal for applications requiring long-term durability in harsh environments. For optimal corrosion resistance, chromium is the preferred metal, especially in automotive, aerospace, and industrial coatings.
Infographic: Magnesium vs Chromium for Corrosion Resistance
 azmater.com
azmater.com