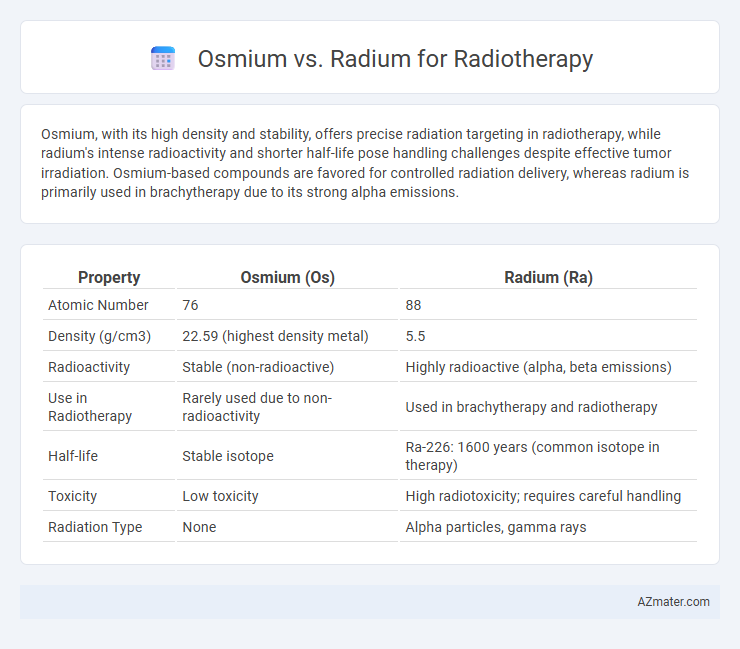
Osmium, with its high density and stability, offers precise radiation targeting in radiotherapy, while radium's intense radioactivity and shorter half-life pose handling challenges despite effective tumor irradiation. Osmium-based compounds are favored for controlled radiation delivery, whereas radium is primarily used in brachytherapy due to its strong alpha emissions.
Table of Comparison
| Property | Osmium (Os) | Radium (Ra) |
|---|---|---|
| Atomic Number | 76 | 88 |
| Density (g/cm3) | 22.59 (highest density metal) | 5.5 |
| Radioactivity | Stable (non-radioactive) | Highly radioactive (alpha, beta emissions) |
| Use in Radiotherapy | Rarely used due to non-radioactivity | Used in brachytherapy and radiotherapy |
| Half-life | Stable isotope | Ra-226: 1600 years (common isotope in therapy) |
| Toxicity | Low toxicity | High radiotoxicity; requires careful handling |
| Radiation Type | None | Alpha particles, gamma rays |
Introduction to Osmium and Radium in Radiotherapy
Osmium and radium are heavy metals studied for their potential applications in radiotherapy due to their unique radioactive properties. Radium-223 is widely recognized and clinically utilized in targeted alpha therapy for conditions like metastatic prostate cancer, leveraging its alpha particle emissions to destroy cancer cells with limited damage to surrounding tissue. Osmium, particularly its isotopes such as osmium-191 and osmium-193, is being explored for novel radiotherapeutic approaches because of its high-density atomic structure and potential to emit therapeutic radiation, offering promising alternatives for precise cancer treatment.
Historical Use of Osmium and Radium in Cancer Treatment
Radium was historically prominent in radiotherapy since its discovery in 1898, utilized extensively for cancer treatment due to its strong alpha and beta radiation emissions. Osmium, although less common, was explored in early 20th-century research for its potential radioisotopes but lacked the widespread clinical adoption that radium achieved. The radioactive properties of radium, along with its availability and effectiveness, established its primary role in early cancer radiotherapy protocols.
Chemical Properties: Osmium vs Radium
Osmium exhibits a high density of 22.59 g/cm3 and exceptional chemical stability, making it resistant to corrosion and ideal for targeted radiotherapy applications requiring durable materials. Radium, as an alkaline earth metal with atomic number 88, is highly radioactive and chemically reactive, forming compounds that readily release alpha particles used in radiation treatments but pose significant handling challenges. The contrasting chemical inertness of osmium versus the intense radioactivity and reactivity of radium directly impacts their suitability and safety profiles in radiotherapy contexts.
Mechanisms of Action in Radiotherapy
Osmium and radium differ significantly in their mechanisms of action within radiotherapy due to their distinct radioactive properties. Osmium isotopes, particularly osmium-191 and osmium-193, emit alpha and beta particles with high linear energy transfer (LET), causing localized DNA double-strand breaks primarily effective for targeting hypoxic tumor cells. Radium-223, a well-known alpha emitter, selectively targets bone metastases by mimicking calcium, delivering high-energy alpha particles that induce complex DNA damage and apoptosis in cancer cells within the bone microenvironment, making it highly effective in treating metastatic bone tumors.
Efficacy Comparison: Treatment Outcomes
Osmium and radium exhibit distinct efficacies in radiotherapy, with radium historically favored for treating certain cancers due to its strong alpha particle emissions and deep tissue penetration. Osmium's isotopes, such as Osmium-191, show potential for more targeted therapy with reduced collateral damage, owing to their precise energy delivery and shorter half-lives. Current comparative studies highlight radium's established success in bone metastases, while osmium-based treatments are being explored for improved outcomes in localized tumors with minimized side effects.
Safety and Toxicity Profiles
Osmium exhibits significantly lower radioactivity and toxicity compared to radium, making it a safer option for radiotherapy applications. Radium's intense radioactivity poses high risks of radiation damage and toxicity to healthy tissues, necessitating strict handling protocols. The controlled radioisotopes of osmium provide targeted therapeutic effects with minimized systemic toxicity, enhancing patient safety during cancer treatment.
Availability and Cost Analysis
Osmium is a rare transition metal with limited availability and extremely high cost, making it impractical for widespread use in radiotherapy despite its high density and potential radiation shielding properties. Radium, historically used in radiotherapy, is more accessible but highly radioactive and expensive due to safety regulations and the need for secure handling and disposal. Cost analysis reveals radium materials are significantly more manageable financially for clinical applications, though emerging alternatives and synthetic isotopes are being explored for optimized balance between availability, cost, and therapeutic efficacy.
Handling and Storage Requirements
Osmium, a dense and stable transition metal, requires stringent handling protocols due to its toxicity and potential to form osmium tetroxide, a highly toxic compound, necessitating storage in airtight containers with proper ventilation controls. Radium, a radioactive alkaline earth metal, demands specialized radiological safety measures, including lead-shielded storage to prevent radiation exposure and strict regulatory compliance to manage its radioactivity hazards. Both elements require trained personnel and robust safety systems, but radium's radioactivity introduces higher risks and complexities compared to osmium's chemical toxicity in radiotherapy applications.
Regulatory and Environmental Considerations
Osmium, particularly in its stable isotope forms, poses fewer regulatory and environmental challenges compared to radium, which is highly radioactive and strictly controlled due to its intense gamma emissions and associated health risks. Radium's long half-life and radiotoxicity necessitate rigorous handling protocols, secure disposal methods, and compliance with agencies like the Nuclear Regulatory Commission (NRC) to prevent environmental contamination. In contrast, osmium's use in radiotherapy requires less stringent regulatory oversight, reducing potential environmental hazards linked to radioactive waste management and enhancing treatment safety profiles.
Future Prospects of Osmium and Radium in Oncology
Osmium isotopes, particularly osmium-191 and osmium-192, show promise for targeted radiotherapy due to their high-density properties and potential for precise alpha particle emission, offering a novel approach to cancer cell eradication with minimal damage to surrounding tissues. Radium-223 dichloride currently leads in clinical use for metastatic prostate cancer by selectively targeting bone metastases via alpha particle therapy, demonstrating improved survival rates and reduced side effects compared to traditional treatments. Future oncology research is likely to focus on developing osmium-based radiopharmaceuticals that leverage its unique chemical stability and radiophysical characteristics, while continuing to optimize radium therapies for broader cancer types and combined modality treatments.
Infographic: Osmium vs Radium for Radiotherapy
 azmater.com
azmater.com