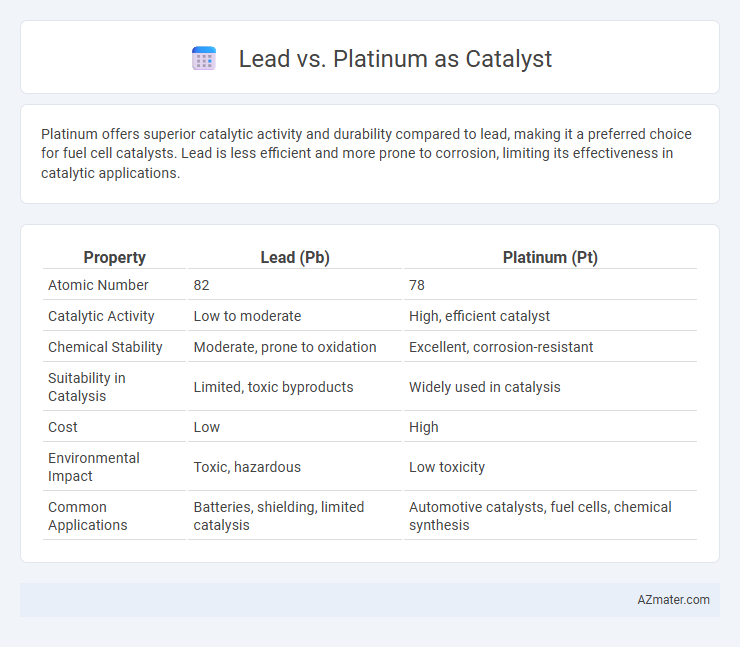
Platinum offers superior catalytic activity and durability compared to lead, making it a preferred choice for fuel cell catalysts. Lead is less efficient and more prone to corrosion, limiting its effectiveness in catalytic applications.
Table of Comparison
| Property | Lead (Pb) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 82 | 78 |
| Catalytic Activity | Low to moderate | High, efficient catalyst |
| Chemical Stability | Moderate, prone to oxidation | Excellent, corrosion-resistant |
| Suitability in Catalysis | Limited, toxic byproducts | Widely used in catalysis |
| Cost | Low | High |
| Environmental Impact | Toxic, hazardous | Low toxicity |
| Common Applications | Batteries, shielding, limited catalysis | Automotive catalysts, fuel cells, chemical synthesis |
Introduction to Catalysts: Lead vs Platinum
Lead and platinum serve distinct roles as catalysts, with platinum being widely recognized for its exceptional catalytic activity and resistance to poisoning in chemical reactions such as hydrogenation and fuel cells. Lead, though less efficient and more toxic, is occasionally used in niche applications where cost-effectiveness outweighs performance, such as in lead-based electrodes or in lead-acid batteries. The choice between lead and platinum catalysts depends on factors like reaction specificity, durability, environmental impact, and economic considerations in industrial and environmental processes.
Chemical Properties of Lead and Platinum
Lead exhibits a relatively low melting point of 327.5degC and demonstrates poor catalytic activity due to its limited ability to adsorb reactant molecules, stemming from its filled 6s and 6p electron orbitals. Platinum possesses a high melting point of 1768degC and excels as a catalyst because of its partially filled d-orbitals, enabling strong adsorption and activation of various reactants through surface electron interactions. The chemical stability and resistance to oxidation of platinum contrast sharply with lead's tendency to form oxides, influencing their respective catalytic lifespans and efficiency.
Catalytic Efficiency: Lead vs Platinum
Platinum catalysts demonstrate significantly higher catalytic efficiency compared to lead, primarily due to their superior ability to facilitate chemical reactions at lower activation energies and higher turnover frequencies. Lead, while less costly, exhibits lower catalytic activity and selectivity, often resulting in slower reaction rates and reduced overall process efficiency. The exceptional surface properties and electronic structure of platinum enable enhanced adsorption and activation of reactants, making it the preferred choice for high-performance catalytic applications.
Environmental Impact of Using Lead and Platinum
Lead used in catalysts poses significant environmental risks due to its toxicity, persistence in ecosystems, and potential to bioaccumulate, leading to soil and water contamination. In contrast, platinum catalysts, while costly, offer greater environmental compatibility as they are less toxic, more stable, and often recyclable, reducing hazardous waste generation. The use of platinum catalysts contributes to cleaner emissions and aligns better with sustainable industrial practices compared to lead-based alternatives.
Cost Comparison: Lead vs Platinum Catalysts
Lead catalysts are significantly cheaper than platinum catalysts, making them a cost-effective option for large-scale industrial applications despite their lower catalytic efficiency. Platinum catalysts, while expensive due to scarcity and complex extraction processes, offer superior catalytic activity and durability, justifying the higher investment in high-performance scenarios. The cost difference between lead and platinum catalysts can be a critical factor in manufacturing decisions, especially when balancing budget constraints with desired catalytic performance.
Applications in Industry: Lead and Platinum
Lead is commonly used as a catalyst in industrial processes such as battery manufacturing and lead-acid batteries due to its cost-effectiveness and availability, but it offers lower catalytic efficiency and durability compared to platinum. Platinum serves as a highly efficient catalyst in automotive catalytic converters, fuel cells, and chemical industries for reactions like hydrogenation, oxidation, and reforming, owing to its superior activity, selectivity, and resistance to corrosion. The choice between lead and platinum catalysts in industry hinges on balancing cost constraints against performance requirements and longevity in specific chemical reactions.
Durability and Longevity of Catalysts
Platinum catalysts exhibit superior durability and longevity compared to lead-based catalysts due to their enhanced resistance to corrosion and sintering under high-temperature operation. Lead catalysts often suffer from faster degradation and poisoning, limiting their operational lifespan in industrial applications. The robust chemical stability of platinum ensures sustained catalytic activity, making it the preferred choice for long-term processes.
Safety Concerns and Toxicity
Lead catalysts pose significant safety concerns due to their high toxicity and potential for environmental contamination, leading to strict regulatory restrictions in many industries. Platinum catalysts are generally safer, exhibiting lower toxicity and reduced environmental impact, making them preferable for applications requiring minimal health risks. Despite platinum's higher cost, its superior safety profile and non-toxic nature make it a more sustainable choice for catalytic processes.
Future Trends in Catalyst Materials
Platinum remains the dominant catalyst material in automotive and industrial applications due to its exceptional activity and durability, but rising costs and supply concerns drive research into alternative elements like lead and lead-based alloys. Emerging trends emphasize developing lead-doped catalysts that offer enhanced selectivity and toxicity reduction while lowering overall platinum usage to meet sustainability and cost-efficiency goals. Advances in nanostructured composites and hybrid catalysts are likely to shape future catalyst materials, balancing performance with environmental regulations and resource availability.
Conclusion: Choosing the Right Catalyst
Platinum catalysts exhibit superior catalytic activity, durability, and resistance to poisoning compared to lead-based catalysts, making them the preferred choice in high-performance applications such as fuel cells and automotive catalytic converters. Lead catalysts, although less expensive, pose significant environmental and health risks due to their toxicity and limited catalytic efficiency, restricting their usage in modern, sustainable technologies. Selecting the right catalyst hinges on balancing performance requirements, environmental impact, and regulatory compliance, with platinum remaining the optimal option for long-term, high-efficiency catalytic processes.
Infographic: Lead vs Platinum for Catalyst
 azmater.com
azmater.com