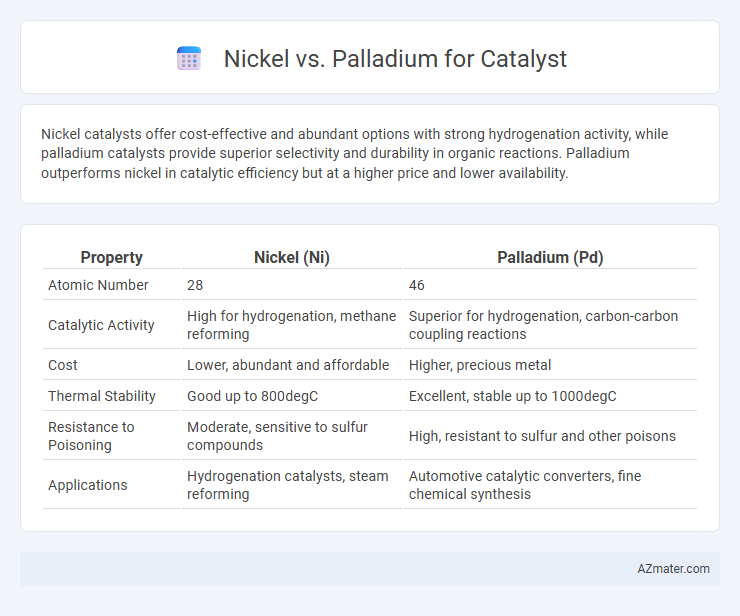
Nickel catalysts offer cost-effective and abundant options with strong hydrogenation activity, while palladium catalysts provide superior selectivity and durability in organic reactions. Palladium outperforms nickel in catalytic efficiency but at a higher price and lower availability.
Table of Comparison
| Property | Nickel (Ni) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 28 | 46 |
| Catalytic Activity | High for hydrogenation, methane reforming | Superior for hydrogenation, carbon-carbon coupling reactions |
| Cost | Lower, abundant and affordable | Higher, precious metal |
| Thermal Stability | Good up to 800degC | Excellent, stable up to 1000degC |
| Resistance to Poisoning | Moderate, sensitive to sulfur compounds | High, resistant to sulfur and other poisons |
| Applications | Hydrogenation catalysts, steam reforming | Automotive catalytic converters, fine chemical synthesis |
Introduction to Nickel and Palladium Catalysts
Nickel and palladium are widely used catalysts in chemical reactions due to their unique properties and effectiveness in enhancing reaction rates. Nickel catalysts are favored for hydrogenation and reforming processes because of their cost-efficiency and ability to activate hydrogen molecules, while palladium catalysts are highly valued for hydrogenation, carbon-carbon coupling, and oxidation reactions due to palladium's exceptional selectivity and stability. Both metals play critical roles in industrial catalysis, with nickel preferred in large-scale applications and palladium dominating in fine chemical synthesis and environmental catalysis.
Chemical Properties: Nickel vs Palladium
Nickel exhibits excellent catalytic activity in hydrogenation due to its strong affinity for hydrogen adsorption and moderate electronegativity, facilitating effective electron transfer during reactions. Palladium, with a higher atomic number and greater d-electron availability, provides superior catalytic selectivity and resistance to poisoning in organic transformations, particularly in carbon-carbon coupling reactions. The distinct electronic configurations and surface energies of nickel and palladium account for their differing catalytic efficiencies and substrate specificities in industrial chemical processes.
Catalytic Efficiency and Performance
Nickel catalysts offer robust catalytic efficiency with high activity in hydrogenation and reforming reactions, making them cost-effective for large-scale industrial applications. Palladium catalysts exhibit superior performance in selective hydrogenation and carbon-carbon coupling reactions due to their high surface reactivity and resistance to poisoning. The choice between nickel and palladium hinges on the specific reaction conditions, desired selectivity, and economic considerations, with palladium often preferred for fine chemical synthesis and nickel favored for bulk processing.
Common Applications in Industry
Nickel catalysts are commonly used in hydrogenation processes, including the production of margarine and the refining of petroleum, due to their cost-effectiveness and high activity. Palladium catalysts excel in automotive catalytic converters and fine chemical synthesis, notably in hydrogenation and carbon-carbon coupling reactions, due to their superior selectivity and resistance to poisoning. Industries rely on nickel for large-scale bulk processes and palladium for precision applications requiring catalytic efficiency and durability.
Cost Comparison: Nickel vs Palladium
Nickel catalysts offer a significantly lower cost compared to palladium, making them a preferred choice in large-scale industrial applications where budget constraints are critical. Palladium, while more expensive, provides higher catalytic activity and selectivity in hydrogenation reactions, often justifying its premium price in fine chemical synthesis. The price difference is substantial, with palladium averaging several hundred dollars per ounce versus nickel's cost, which is typically a fraction of that, influencing catalyst selection based on economic considerations.
Environmental Impact and Sustainability
Nickel and palladium catalysis differ significantly in environmental impact and sustainability; nickel is abundant and less expensive, making it a more sustainable choice with lower ecological footprint, while palladium's rarity and high demand lead to intensive mining practices that result in greater habitat disruption and energy consumption. Nickel catalysts also tend to be more recyclable and less toxic, reducing long-term environmental hazards compared to palladium, which can pose heavy metal contamination risks. Transitioning to nickel-based catalysts supports circular economy principles and helps mitigate the environmental challenges associated with precious metal extraction and waste management.
Catalyst Lifespan and Reusability
Nickel catalysts generally exhibit longer lifespan and enhanced reusability compared to palladium catalysts due to their higher resistance to sintering and poisoning during chemical reactions. Palladium catalysts, while offering superior activity and selectivity in hydrogenation processes, tend to degrade faster under harsh reaction conditions, limiting their operational durability. Optimal catalyst design often involves stabilizing nickel elements with supports or alloying palladium to improve longevity and recyclability in industrial applications.
Challenges and Limitations
Nickel catalysts face challenges such as lower resistance to sintering and coke formation, which reduce their long-term stability and effectiveness in hydrogenation reactions compared to palladium. Palladium offers superior catalytic activity and selectivity, but its high cost and susceptibility to poisoning by sulfur compounds limit its practical applications. The trade-off between nickel's affordability and palladium's performance necessitates ongoing research to enhance catalyst durability and cost-efficiency in industrial processes.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology have demonstrated Nickel's growing role as a cost-effective alternative to Palladium in hydrogenation and carbon-carbon coupling reactions, leveraging its abundance and lower price point. Innovations in nanoparticle design and alloy formation have enhanced Nickel's catalytic activity and selectivity, reducing dependence on scarce Palladium resources. Palladium catalysts continue to dominate in high-precision applications due to their superior tolerance and efficiency, but breakthroughs in Nickel-based catalysts are rapidly closing the performance gap.
Choosing the Right Catalyst: Key Considerations
Nickel offers cost-effective catalytic activity suitable for hydrogenation reactions, but palladium provides superior selectivity and stability in complex organic transformations. Catalyst choice depends on reaction type, desired product purity, and operational conditions such as temperature and pressure. Evaluating catalyst efficiency, lifespan, and compatibility with substrates ensures optimal performance in industrial and laboratory processes.
Infographic: Nickel vs Palladium for Catalyst
 azmater.com
azmater.com