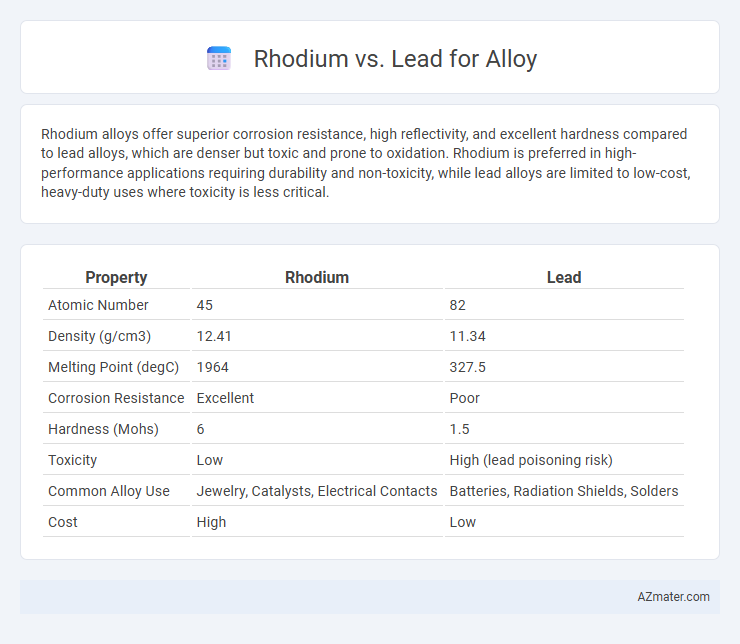
Rhodium alloys offer superior corrosion resistance, high reflectivity, and excellent hardness compared to lead alloys, which are denser but toxic and prone to oxidation. Rhodium is preferred in high-performance applications requiring durability and non-toxicity, while lead alloys are limited to low-cost, heavy-duty uses where toxicity is less critical.
Table of Comparison
| Property | Rhodium | Lead |
|---|---|---|
| Atomic Number | 45 | 82 |
| Density (g/cm3) | 12.41 | 11.34 |
| Melting Point (degC) | 1964 | 327.5 |
| Corrosion Resistance | Excellent | Poor |
| Hardness (Mohs) | 6 | 1.5 |
| Toxicity | Low | High (lead poisoning risk) |
| Common Alloy Use | Jewelry, Catalysts, Electrical Contacts | Batteries, Radiation Shields, Solders |
| Cost | High | Low |
Introduction to Rhodium and Lead Alloys
Rhodium alloys exhibit exceptional corrosion resistance, high melting points, and excellent hardness, making them valuable in catalytic converters and electrical contacts. Lead alloys, known for their low melting points, high density, and malleability, are widely used in batteries, radiation shielding, and soldering applications. Understanding the distinct physical and chemical properties of rhodium and lead alloys is crucial for selecting appropriate materials in industrial and technological applications.
Chemical Properties: Rhodium vs Lead
Rhodium exhibits exceptional chemical resistance due to its inertness and resistance to corrosion, making it ideal for alloys requiring high durability and oxidation resistance. Lead, in contrast, is chemically soft and highly reactive with acids, displaying significant susceptibility to oxidation and tarnishing, which limits its alloy applications. The stark chemical property differences--rhodium's noble metal characteristics versus lead's reactive nature--dictate their distinct roles in alloy formulations, favoring rhodium in high-performance and lead in low-cost or radiation shielding alloys.
Physical Characteristics Comparison
Rhodium exhibits exceptional hardness and high melting point around 1964degC, making it highly resistant to wear and corrosion, whereas Lead has a much lower melting point of 327.5degC and is soft and malleable. The density of Rhodium is approximately 12.41 g/cm3, significantly higher than Lead's 11.34 g/cm3, contributing to Rhodium's superior structural strength in alloys. Thermal and electrical conductivity of Rhodium surpass Lead, enhancing its suitability for high-performance and precision applications in alloy compositions.
Alloying Behavior and Compatibility
Rhodium exhibits excellent alloying behavior with precious metals like platinum and palladium due to its high melting point and noble character, enhancing corrosion resistance and hardness without compromising ductility. Lead, in contrast, has limited compatibility in high-performance alloys because of its low melting point and tendency to segregate, negatively affecting mechanical strength and thermal stability. Rhodium's superior miscibility and chemical inertness make it ideal for durable, high-value alloys, whereas lead is primarily suitable for low-temperature applications requiring malleability and weight.
Applications in Industry
Rhodium is widely used in automotive catalytic converters due to its exceptional corrosion resistance and ability to efficiently reduce harmful nitrogen oxides, making it crucial for emission control systems. Lead alloys find significant application in battery grids and radiation shielding, leveraging lead's high density and excellent durability in harsh environments. The choice between rhodium and lead alloys depends on the specific industrial requirements for corrosion resistance, toxicity, and mechanical strength.
Corrosion Resistance and Durability
Rhodium alloys exhibit exceptional corrosion resistance and superior durability compared to lead alloys, making them ideal for harsh environments and applications requiring long-lasting performance. Lead alloys, while cost-effective, are prone to oxidation and degrade more quickly under corrosive conditions, limiting their lifespan and structural integrity. The high melting point and chemical inertness of rhodium contribute significantly to its enhanced durability in industrial and jewelry applications.
Mechanical Strength and Hardness
Rhodium alloys exhibit significantly higher mechanical strength and hardness compared to lead alloys, making them ideal for applications requiring durability and wear resistance. Rhodium's hardness typically exceeds 6.0 on the Mohs scale, whereas lead's softness is around 1.5, resulting in vastly different performance under stress and abrasion. The superior tensile strength and hardness of rhodium alloys contribute to enhanced structural integrity, whereas lead's low mechanical properties limit its use in load-bearing or high-stress environments.
Cost and Availability Factors
Rhodium is significantly more expensive than lead due to its rarity and high demand in automotive and catalytic converter industries, with prices often exceeding $10,000 per ounce. Lead is much more abundant and affordable, commonly priced under $2 per pound, making it a cost-effective metal for large-scale alloy production. Availability constraints of rhodium limit its use to specialized applications, whereas lead's widespread availability supports broader industrial use.
Environmental and Health Considerations
Rhodium, a rare and highly durable metal, poses minimal environmental and health risks due to its resistance to corrosion and toxicity, making it a safer choice for alloy applications compared to lead. Lead, widely known for its detrimental effects on human health, including neurotoxicity and chronic poisoning, also presents significant environmental contamination risks through soil and water pollution. Regulatory restrictions on lead use in alloys emphasize the shift toward rhodium and other non-toxic metals to ensure safer industrial and consumer products.
Choosing Between Rhodium and Lead for Specific Alloys
Rhodium offers superior corrosion resistance, high reflectivity, and exceptional hardness, making it ideal for high-performance, decorative, and electrical contact alloys. Lead, valued for its malleability, low melting point, and excellent vibration damping properties, is often preferred in alloys requiring ease of machining and enhanced impact resistance. Selecting between rhodium and lead depends on the application's demands for durability, conductivity, and environmental considerations, especially since lead's toxicity restricts its use in consumer products.
Infographic: Rhodium vs Lead for Alloy
 azmater.com
azmater.com