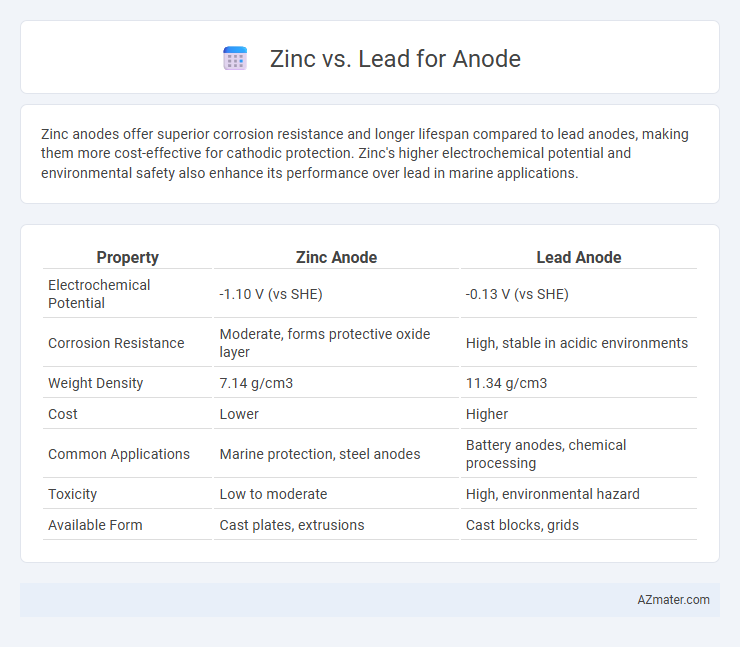
Zinc anodes offer superior corrosion resistance and longer lifespan compared to lead anodes, making them more cost-effective for cathodic protection. Zinc's higher electrochemical potential and environmental safety also enhance its performance over lead in marine applications.
Table of Comparison
| Property | Zinc Anode | Lead Anode |
|---|---|---|
| Electrochemical Potential | -1.10 V (vs SHE) | -0.13 V (vs SHE) |
| Corrosion Resistance | Moderate, forms protective oxide layer | High, stable in acidic environments |
| Weight Density | 7.14 g/cm3 | 11.34 g/cm3 |
| Cost | Lower | Higher |
| Common Applications | Marine protection, steel anodes | Battery anodes, chemical processing |
| Toxicity | Low to moderate | High, environmental hazard |
| Available Form | Cast plates, extrusions | Cast blocks, grids |
Introduction to Anode Materials: Zinc vs Lead
Zinc anodes offer superior corrosion resistance and higher electrochemical efficiency compared to lead anodes, making them ideal for marine and cathodic protection applications. Lead anodes, while less expensive and easier to cast, exhibit lower performance and faster degradation in aggressive environments. The choice between zinc and lead anodes depends on the specific requirements for durability, conductivity, and environmental conditions.
Chemical Properties of Zinc and Lead Anodes
Zinc anodes exhibit a standard electrode potential of -0.76 V, making them highly effective sacrificial anodes in cathodic protection systems due to their stable oxidation and resistance to passivation. Lead anodes possess a higher density and exhibit a standard electrode potential of -0.13 V, resulting in lower anodic efficiency and a more sluggish dissolution process compared to zinc. The chemical stability and ionization tendencies of zinc allow for consistent electron flow, while lead's heavier atomic structure and lower reactivity lead to reduced electrical conductivity and slower anode consumption.
Electrochemical Performance Comparison
Zinc anodes exhibit higher electrochemical efficiency and corrosion resistance compared to lead anodes, making them preferable for sacrificial anode applications in cathodic protection systems. Zinc's standard electrode potential (-0.76 V vs SHE) allows it to effectively protect steel structures by sacrificing itself to oxidation, whereas lead anodes, with a less negative potential (-0.13 V vs SHE), offer lower driving voltage and reduced protection capacity. The superior current capacity and longer lifespan of zinc anodes under various environmental conditions enhance their performance over lead anodes in marine and underground corrosion mitigation.
Corrosion Resistance: Zinc vs Lead
Zinc anodes exhibit superior corrosion resistance compared to lead anodes due to their higher electrochemical activity, which promotes consistent galvanic protection in marine and industrial environments. Zinc forms a stable oxide layer that enhances its durability, whereas lead anodes are more prone to rapid surface degradation and passivation under anodic polarization. This makes zinc the preferred choice for long-term corrosion resistance in sacrificial anode applications.
Environmental Impact of Zinc and Lead Anodes
Zinc anodes exhibit lower environmental toxicity compared to lead anodes, as zinc is less harmful to aquatic life and does not bioaccumulate in ecosystems. Lead anodes release highly toxic lead ions, which pose significant risks to water quality, aquatic organisms, and soil contamination, leading to long-term ecological damage. The use of zinc anodes aligns better with environmental regulations due to their reduced hazardous waste production and easier recyclability.
Cost Analysis: Zinc Anodes vs Lead Anodes
Zinc anodes typically offer a more cost-effective solution compared to lead anodes due to their lower material costs and longer lifespan in marine environments, reducing replacement frequency and maintenance expenses. Lead anodes, while less expensive upfront, often incur higher operational costs because of their shorter service life and less efficient corrosion protection. Evaluating the total cost of ownership reveals zinc anodes provide better economic value in most cathodic protection applications.
Durability and Lifespan of Each Anode Type
Zinc anodes exhibit superior durability in marine environments due to their slower consumption rate and excellent corrosion resistance, resulting in a longer operational lifespan compared to lead anodes. Lead anodes tend to degrade faster under similar conditions, limiting their effectiveness and requiring more frequent replacements. The extended lifespan of zinc anodes reduces maintenance costs and downtime in cathodic protection systems.
Application Suitability: Where Each Anode Excels
Zinc anodes excel in marine applications due to their superior corrosion resistance in seawater environments, making them ideal for ship hulls and offshore structures. Lead anodes are preferred in industrial electroplating and battery manufacturing for their stability and effectiveness in acidic or alkaline electrolytes. Selecting the appropriate anode depends on the specific environment, with zinc outperforming in chloride-rich waters and lead providing consistent performance in controlled electrochemical processes.
Safety Considerations for Handling and Use
Zinc anodes offer enhanced safety due to lower toxicity compared to lead anodes, reducing health risks during handling and disposal. Lead anodes require strict protective measures to prevent lead exposure, which can cause serious health issues such as neurological and kidney damage. Proper use and handling protocols for zinc anodes minimize environmental contamination and occupational hazards, making zinc a safer choice in anode applications.
Future Trends in Anode Material Selection
Zinc anodes are gaining traction due to their environmental benefits, cost-effectiveness, and superior corrosion resistance compared to lead anodes, which face increasing regulatory restrictions due to toxicity. Emerging research emphasizes alloying zinc with other metals to enhance durability and electrochemical performance in marine and industrial applications. Future trends indicate a shift towards sustainable, high-efficiency zinc-based anodes driven by stricter environmental policies and advancements in material science.
Infographic: Zinc vs Lead for Anode
 azmater.com
azmater.com