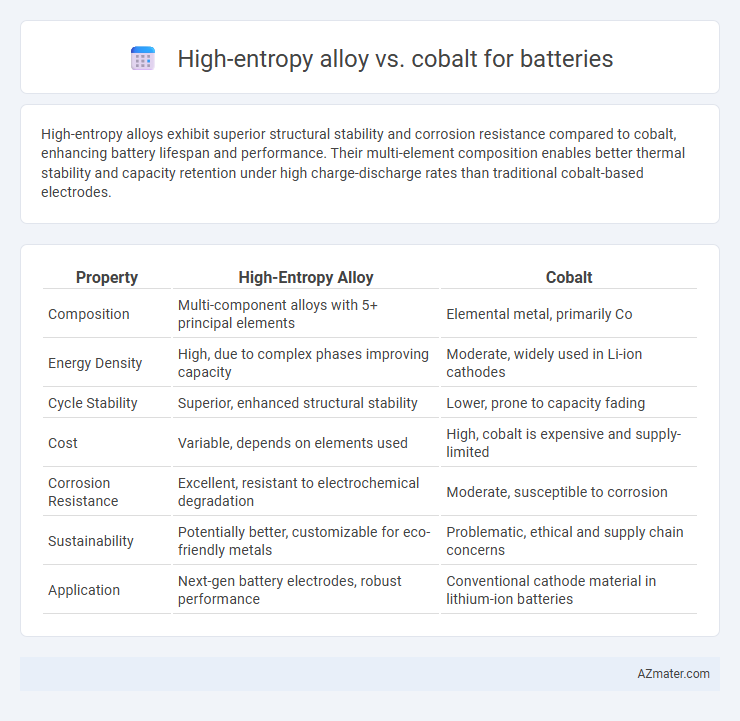
High-entropy alloys exhibit superior structural stability and corrosion resistance compared to cobalt, enhancing battery lifespan and performance. Their multi-element composition enables better thermal stability and capacity retention under high charge-discharge rates than traditional cobalt-based electrodes.
Table of Comparison
| Property | High-Entropy Alloy | Cobalt |
|---|---|---|
| Composition | Multi-component alloys with 5+ principal elements | Elemental metal, primarily Co |
| Energy Density | High, due to complex phases improving capacity | Moderate, widely used in Li-ion cathodes |
| Cycle Stability | Superior, enhanced structural stability | Lower, prone to capacity fading |
| Cost | Variable, depends on elements used | High, cobalt is expensive and supply-limited |
| Corrosion Resistance | Excellent, resistant to electrochemical degradation | Moderate, susceptible to corrosion |
| Sustainability | Potentially better, customizable for eco-friendly metals | Problematic, ethical and supply chain concerns |
| Application | Next-gen battery electrodes, robust performance | Conventional cathode material in lithium-ion batteries |
Introduction: Emerging Materials in Battery Technology
High-entropy alloys (HEAs) and cobalt are prominent materials in advanced battery technology, each offering distinct electrochemical properties. HEAs exhibit remarkable structural stability and corrosion resistance due to their multi-element compositions, enhancing battery lifespan and performance. Cobalt remains a critical component in lithium-ion batteries for its high energy density and conductivity, but challenges related to supply risk and cost drive research toward HEA-based alternatives.
What Are High-Entropy Alloys?
High-entropy alloys (HEAs) are advanced materials composed of five or more principal elements mixed in near-equal atomic ratios, creating a highly stable and disordered solid solution. This unique composition grants HEAs exceptional mechanical strength, corrosion resistance, and thermal stability, making them promising candidates for battery electrode materials. In battery technology, HEAs offer improved electrochemical performance and durability compared to traditional cobalt-based electrodes, enhancing energy density and cycle life.
Cobalt: A Key Material in Current Batteries
Cobalt is a critical component in lithium-ion batteries, known for its ability to improve energy density and enhance battery stability, making it indispensable in electric vehicle and portable electronics markets. Despite concerns over cost and ethical sourcing, cobalt's excellent electrochemical properties contribute significantly to battery performance by enabling longer cycle life and higher voltage. Research continues to optimize cobalt content, balancing efficiency with sustainability to maintain its central role in current battery technologies.
Structural Differences: High-Entropy Alloy vs. Cobalt
High-entropy alloys (HEAs) consist of multiple principal elements, typically five or more, creating a complex, disordered atomic structure that enhances mechanical stability and corrosion resistance in battery applications. In contrast, cobalt is a single-element metal with a well-ordered crystalline structure, providing high electrical conductivity but less structural adaptability under battery cycling conditions. The multi-element composition of HEAs results in lattice distortion that improves ionic diffusion and mitigates volume expansion, unlike pure cobalt electrodes which can suffer from structural degradation and capacity fading.
Energy Density Comparison
High-entropy alloys exhibit superior energy density compared to cobalt-based materials due to their multi-element composition, which enhances electrochemical performance and structural stability in batteries. These alloys enable higher capacity retention and improved cycle life by mitigating degradation mechanisms that commonly affect cobalt electrodes. Consequently, high-entropy alloy batteries demonstrate a significant advantage in energy density, making them promising candidates for next-generation energy storage technologies.
Durability and Lifespan Analysis
High-entropy alloys (HEAs) exhibit superior durability and extended lifespan compared to cobalt-based materials in battery applications due to their multi-element composition, which enhances resistance to corrosion, mechanical degradation, and thermal stress. Cobalt, while offering high conductivity and energy density, tends to suffer from rapid capacity fading and structural instability under prolonged cycling. Studies reveal that HEAs maintain structural integrity and electrochemical performance over thousands of charge-discharge cycles, making them a promising alternative for next-generation durable battery electrodes.
Cost and Resource Availability
High-entropy alloys (HEAs) offer significant cost advantages over cobalt due to their utilization of abundant and less expensive elements, reducing dependence on scarce cobalt resources. Cobalt's high market price and geopolitical supply risks, primarily from limited mining regions, make its usage in batteries costly and vulnerable to supply chain disruptions. HEAs provide a more sustainable and economically viable alternative for battery materials by leveraging diverse, readily available metals.
Environmental Impact and Sustainability
High-entropy alloys (HEAs) offer promising environmental benefits over cobalt in battery applications due to their potential for reduced reliance on scarce and conflict-associated cobalt resources. HEAs, composed of multiple principal elements, can enhance battery durability and recyclability, minimizing resource depletion and toxic waste generation. Advancements in HEA technology contribute to sustainable battery development by lowering ecological footprints and promoting circular material usage.
Performance in Real-world Applications
High-entropy alloys (HEAs) exhibit superior corrosion resistance and structural stability compared to cobalt in battery applications, enhancing cycle life and safety under real-world operating conditions. HEAs improve capacity retention and reduce degradation rates, making them promising for high-performance energy storage systems, particularly in electric vehicles and renewable energy integration. Cobalt-based materials, while established, face challenges such as cost, toxicity, and limited resource availability, which HEAs help to mitigate through their tunable multi-element composition and improved electrochemical performance.
Future Prospects: High-Entropy Alloy vs. Cobalt
High-entropy alloys (HEAs) exhibit exceptional structural stability and electrochemical performance, making them promising candidates for next-generation battery electrodes compared to traditional cobalt-based materials. HEAs offer enhanced corrosion resistance, tunable properties through multi-element compositions, and reduced reliance on scarce and geopolitically sensitive cobalt resources. Future prospects include improving energy density, cycle life, and sustainability in batteries by leveraging the unique atomic-scale interactions and disorder in HEAs over conventional cobalt electrodes.
Infographic: High-entropy alloy vs Cobalt for Battery
 azmater.com
azmater.com