Hafnium offers superior corrosion resistance and high melting points suitable for aerospace and nuclear metallurgy, whereas calcium serves primarily as a deoxidizer and desulfurizer in steelmaking due to its reactive properties. Hafnium's high density and strength at elevated temperatures make it ideal for high-performance alloys, contrasting with calcium's role in improving metal cleanliness and grain refinement.
Table of Comparison
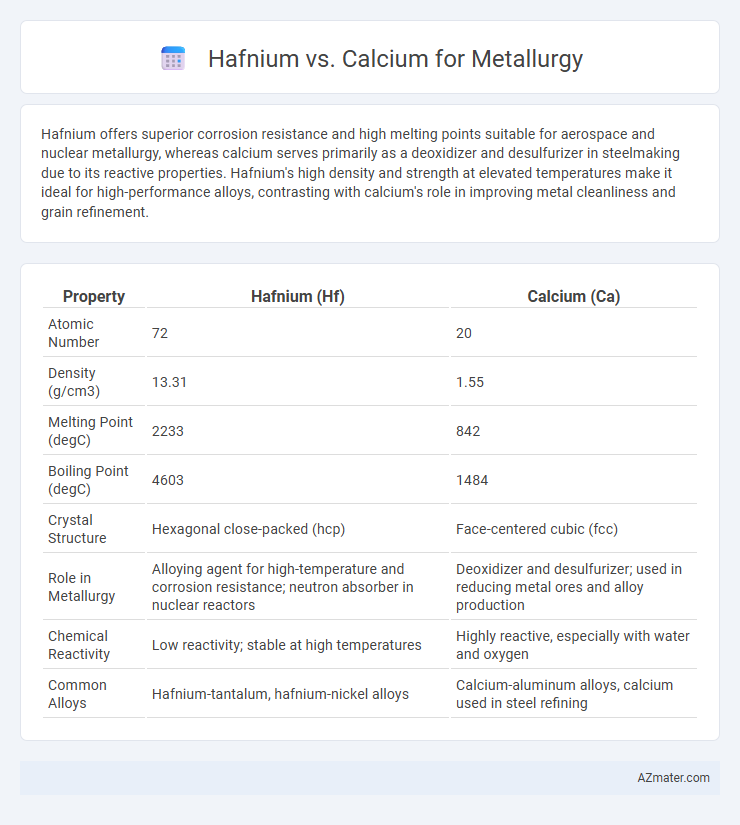
| Property | Hafnium (Hf) | Calcium (Ca) |
|---|---|---|
| Atomic Number | 72 | 20 |
| Density (g/cm3) | 13.31 | 1.55 |
| Melting Point (degC) | 2233 | 842 |
| Boiling Point (degC) | 4603 | 1484 |
| Crystal Structure | Hexagonal close-packed (hcp) | Face-centered cubic (fcc) |
| Role in Metallurgy | Alloying agent for high-temperature and corrosion resistance; neutron absorber in nuclear reactors | Deoxidizer and desulfurizer; used in reducing metal ores and alloy production |
| Chemical Reactivity | Low reactivity; stable at high temperatures | Highly reactive, especially with water and oxygen |
| Common Alloys | Hafnium-tantalum, hafnium-nickel alloys | Calcium-aluminum alloys, calcium used in steel refining |
Introduction to Hafnium and Calcium in Metallurgy
Hafnium in metallurgy is prized for its exceptional corrosion resistance and high melting point, making it ideal for control rods in nuclear reactors and superalloys in aerospace applications, whereas calcium serves as a strong deoxidizer and desulfurizer in steel production, improving metal quality by removing impurities. Hafnium's ability to form stable compounds enhances alloy strength and thermal stability, while calcium's reactivity helps refine molten metals and reduce inclusions, optimizing material performance. Both elements contribute uniquely to metallurgical processes, with hafnium focusing on high-performance alloy enhancement and calcium on purification and structural integrity of metals.
Chemical Properties and Reactivity
Hafnium exhibits high corrosion resistance and low thermal neutron capture cross-section, making it valuable in nuclear reactors and high-temperature alloys, whereas calcium is highly reactive, readily oxidizing and reacting with water to form calcium hydroxide. Chemically, hafnium's stable +4 oxidation state contrasts with calcium's +2 state, influencing their metallurgical applications and alloying behavior. Hafnium's dense atomic structure and resistance to chemical attack enable durability in extreme environments, while calcium's reactivity is leveraged for deoxidizing and desulfurizing metals during refining.
Extraction and Refining Processes
Hafnium extraction typically involves solvent extraction and ion exchange techniques to separate it from zirconium due to their chemical similarity, making the refining process complex and energy-intensive. In contrast, calcium is primarily obtained through the electrolysis of molten calcium chloride, a simpler and more cost-effective refining process. The distinct extraction methods reflect hafnium's rarity and refractory nature compared to calcium's abundant and reactive metallic properties in metallurgy.
Metallurgical Roles and Applications
Hafnium in metallurgy is primarily valued for its high melting point, corrosion resistance, and ability to form stable alloys with nickel and titanium, making it essential in aerospace turbine engines and nuclear reactors. Calcium serves as a powerful deoxidizer and desulfurizer in steelmaking, improving the quality and mechanical properties of steel by reducing impurities and modifying the shape of non-metallic inclusions. The distinct roles of hafnium and calcium highlight their importance in enhancing alloy performance and steel purity, respectively, tailoring metals for high-stress and high-temperature applications.
Influence on Alloy Strength and Performance
Hafnium significantly enhances alloy strength and high-temperature performance due to its high melting point and excellent corrosion resistance, making it valuable in superalloys for aerospace applications. Calcium, while beneficial as a deoxidizer and grain refiner, contributes less directly to mechanical strength but improves castability and ductility in steel alloys. The presence of hafnium in alloys supports superior creep resistance and fatigue strength, whereas calcium primarily optimizes microstructure and workability.
Cost and Availability in Industrial Markets
Hafnium, a rare transition metal primarily sourced as a byproduct of zirconium refinement, is significantly more expensive than calcium due to its limited availability and complex extraction process. Calcium, abundant in the Earth's crust and easily extracted from limestone and other minerals, remains a cost-effective choice for large-scale metallurgical applications. Industrial markets favor calcium for cost-sensitive processes, while hafnium's high price limits its use to specialized applications requiring corrosion resistance and high melting points.
Environmental Impact and Safety Considerations
Hafnium in metallurgy offers superior corrosion resistance and high-temperature stability but poses significant environmental disposal challenges due to its rarity and complex extraction processes, which can lead to toxic waste generation. Calcium, widely used as a reducing agent in metal production, is abundant and more environmentally benign, yet it requires careful handling due to its reactive nature, especially with water, posing safety hazards. Selecting between hafnium and calcium involves balancing hafnium's environmental extraction impact and calcium's reactive safety risks to optimize sustainable metallurgical practices.
Compatibility with Other Metals
Hafnium exhibits excellent compatibility with refractory metals such as zirconium, titanium, and niobium due to its high melting point and corrosion resistance, making it ideal for high-temperature alloy applications. Calcium, being highly reactive and possessing a lower melting point, is primarily used as a deoxidizer or desulfurizer in steelmaking rather than as an alloying element, limiting its direct compatibility with most structural metals. Metallurgically, hafnium's stable intermetallic compounds enhance alloy strength and durability, whereas calcium's role is more chemical modification than structural integration.
Case Studies of Industrial Usage
Hafnium demonstrates exceptional corrosion resistance and stability at high temperatures, making it ideal for aerospace and nuclear reactor components, as evidenced by its use in control rods and high-temperature alloys. Calcium is predominantly applied in metallurgy as a deoxidizer and desulfurizing agent in steelmaking, improving metal quality by reducing impurities, supported by case studies from the steel industry. Comparative industrial usage reveals hafnium's superiority in high-performance, high-temperature applications, while calcium excels in refining processes for structural steel production.
Future Trends in Metallurgical Applications
Hafnium's superior corrosion resistance and high melting point position it as a key material in advanced aerospace and nuclear reactor components, surpassing calcium's limited thermal stability. Emerging metallurgical applications increasingly favor hafnium alloys for their exceptional strength-to-weight ratio and neutron absorption properties, critical for next-generation reactors and high-performance turbines. Research into hafnium-calcium composites aims to blend calcium's low density with hafnium's durability, potentially revolutionizing lightweight, high-strength materials in future metallurgical innovations.
Infographic: Hafnium vs Calcium for Metallurgy
 azmater.com
azmater.com