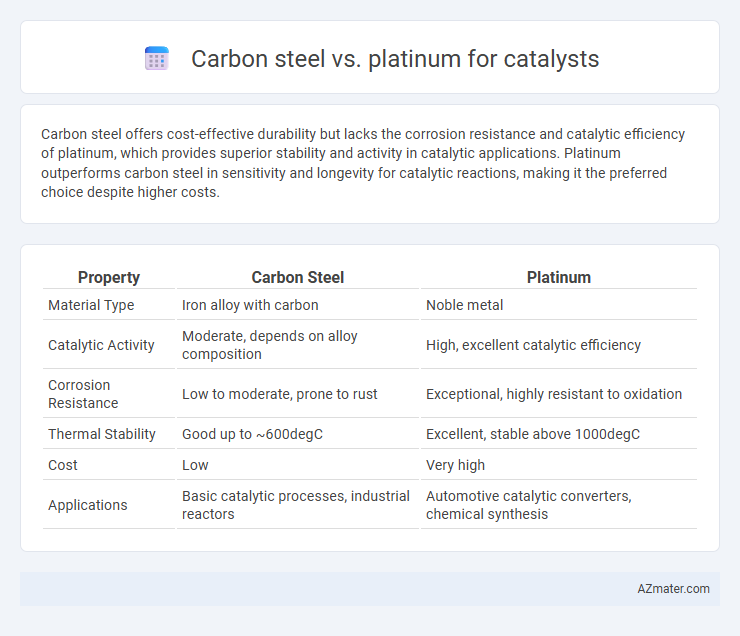
Carbon steel offers cost-effective durability but lacks the corrosion resistance and catalytic efficiency of platinum, which provides superior stability and activity in catalytic applications. Platinum outperforms carbon steel in sensitivity and longevity for catalytic reactions, making it the preferred choice despite higher costs.
Table of Comparison
| Property | Carbon Steel | Platinum |
|---|---|---|
| Material Type | Iron alloy with carbon | Noble metal |
| Catalytic Activity | Moderate, depends on alloy composition | High, excellent catalytic efficiency |
| Corrosion Resistance | Low to moderate, prone to rust | Exceptional, highly resistant to oxidation |
| Thermal Stability | Good up to ~600degC | Excellent, stable above 1000degC |
| Cost | Low | Very high |
| Applications | Basic catalytic processes, industrial reactors | Automotive catalytic converters, chemical synthesis |
Introduction to Catalysts: Carbon Steel vs Platinum
Carbon steel and platinum serve distinctly different roles as catalysts due to their unique material properties and catalytic activity. Platinum, a noble metal with exceptional chemical stability and high catalytic efficiency, is widely used in automotive catalytic converters and chemical reactions requiring precise control and durability. Carbon steel, while less active catalytically, offers cost-effective structural support and is often employed in applications where mechanical strength outweighs catalytic performance.
Chemical Properties: Carbon Steel and Platinum
Carbon steel exhibits moderate corrosion resistance but can oxidize easily in acidic or high-temperature environments, limiting its catalytic stability. Platinum, a noble metal, demonstrates exceptional chemical inertness, high resistance to oxidation, and superior catalytic activity, especially in hydrogenation and oxidation reactions. These chemical properties make platinum a preferred catalyst in harsh chemical processes, whereas carbon steel often requires protective coatings to enhance durability.
Catalytic Efficiency: A Comparative Analysis
Platinum catalysts exhibit significantly higher catalytic efficiency than carbon steel due to their superior surface reactivity and resistance to oxidation. Carbon steel, while cost-effective, often suffers from surface degradation and lower active site availability, limiting its catalytic performance. The inherent inertness and durability of platinum metals enable sustained catalytic activity and selectivity in diverse chemical reactions, making them the preferred choice for high-efficiency catalytic applications.
Cost Considerations: Carbon Steel vs Platinum
Carbon steel offers a significantly lower cost compared to platinum, making it an economically attractive choice for large-scale catalytic applications. While platinum exhibits superior catalytic efficiency and resistance to corrosion, its high market price and limited availability drive up the overall expense. Cost sensitivity in industrial catalyst selection often favors carbon steel when budget constraints outweigh the benefits of platinum's performance.
Durability and Longevity in Catalytic Applications
Platinum catalysts exhibit superior durability and longevity compared to carbon steel due to their exceptional resistance to corrosion and high-temperature stability in catalytic applications. Carbon steel often suffers from oxidation and degradation, leading to shorter operational lifespans under harsh chemical environments. The enhanced catalytic efficiency and resilience of platinum ensure prolonged activity and reduced maintenance costs in industrial processes.
Environmental Impact and Sustainability
Carbon steel catalysts offer advantages in sustainability due to their lower environmental footprint during extraction and processing compared to platinum, which requires intensive mining and refining with significant ecological disruption. Platinum catalysts, while highly efficient and durable, present challenges in recycling and resource depletion due to their rarity and high energy consumption involved in recovery processes. Opting for carbon steel-based catalysts can reduce carbon emissions and resource scarcity, supporting greener industrial applications and long-term environmental sustainability.
Industrial Applications: Where Each Catalyst Excels
Carbon steel catalysts excel in industrial applications requiring cost-effective, durable materials with moderate catalytic activity, particularly in large-scale chemical reactors and synthesis processes where structural integrity under high pressure is essential. Platinum catalysts outperform carbon steel in precision-demanding industries such as automotive catalytic converters and fine chemical production, offering superior resistance to poisoning and exceptional catalytic efficiency in oxidation and hydrogenation reactions. The choice between carbon steel and platinum catalysts hinges on balancing economic constraints with performance needs specific to industrial processes and operational environments.
Catalytic Reaction Types: Suitability of Each Material
Carbon steel catalysts excel in hydrogenation and dehydrogenation reactions due to their strong affinity for hydrogen atoms and robust mechanical properties. Platinum catalysts are highly suitable for oxidation, reforming, and isomerization reactions, offering superior resistance to poisoning and exceptional catalytic activity across various reaction conditions. The choice between carbon steel and platinum depends on the specific catalytic reaction type, operating temperature, and desired selectivity, with platinum preferred for high-precision and versatile catalytic processes.
Innovations and Advancements in Catalyst Materials
Innovations in catalyst materials have led to significant advancements in the use of carbon steel and platinum, with platinum showing superior catalytic efficiency and resistance to corrosion in industrial applications. Recent developments in nanotechnology have enhanced the catalytic surface area of platinum, enabling improved reaction rates and selectivity in chemical processes. Meanwhile, carbon steel hybrids integrated with advanced coatings are being explored for cost-effective catalytic support structures, balancing durability and performance in large-scale operations.
Future Trends: Evolving Uses of Carbon Steel and Platinum in Catalysis
Carbon steel and platinum continue to shape the future of catalysis with their distinct advantages in cost efficiency and catalytic performance. Innovations in carbon steel catalysts emphasize enhanced durability and eco-friendly production methods, making them increasingly viable for large-scale industrial applications. Platinum remains essential in high-precision catalytic processes due to its superior activity and resistance to poisoning, driving ongoing research toward optimizing its use in fuel cells and sustainable energy technologies.
Infographic: Carbon steel vs Platinum for Catalyst
 azmater.com
azmater.com