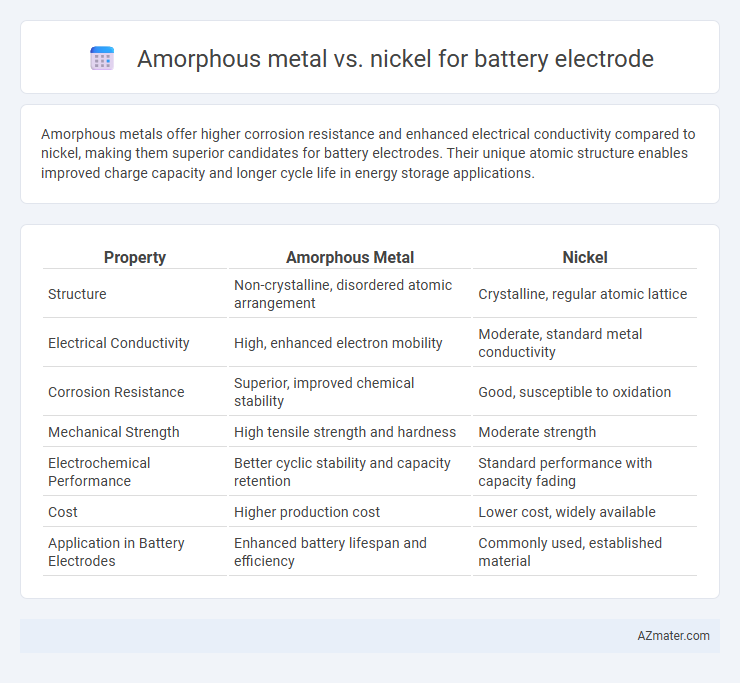
Amorphous metals offer higher corrosion resistance and enhanced electrical conductivity compared to nickel, making them superior candidates for battery electrodes. Their unique atomic structure enables improved charge capacity and longer cycle life in energy storage applications.
Table of Comparison
| Property | Amorphous Metal | Nickel |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Crystalline, regular atomic lattice |
| Electrical Conductivity | High, enhanced electron mobility | Moderate, standard metal conductivity |
| Corrosion Resistance | Superior, improved chemical stability | Good, susceptible to oxidation |
| Mechanical Strength | High tensile strength and hardness | Moderate strength |
| Electrochemical Performance | Better cyclic stability and capacity retention | Standard performance with capacity fading |
| Cost | Higher production cost | Lower cost, widely available |
| Application in Battery Electrodes | Enhanced battery lifespan and efficiency | Commonly used, established material |
Introduction to Battery Electrode Materials
Amorphous metals exhibit a disordered atomic structure that enhances electrochemical performance by providing higher capacity and improved cycling stability compared to crystalline nickel. Nickel, a traditional battery electrode material, offers good conductivity and mechanical strength but often suffers from limited active surface area and degradation over repeated cycles. Advancements in amorphous metal electrodes focus on maximizing ion diffusion and minimizing structural fatigue, making them promising candidates for next-generation energy storage devices.
What Are Amorphous Metals?
Amorphous metals, also known as metallic glasses, are non-crystalline solids characterized by a disordered atomic structure, which contrasts with the highly ordered lattice found in crystalline metals like nickel. This unique structure imparts superior properties such as higher strength, enhanced corrosion resistance, and improved electrochemical behavior, making them promising candidates for battery electrodes. Compared to nickel, amorphous metals offer increased capacity retention and faster charge-discharge rates due to their uniform atomic arrangement and lack of grain boundaries.
Nickel as a Conventional Electrode Material
Nickel is widely recognized as a conventional electrode material in battery technology due to its excellent electrical conductivity, corrosion resistance, and stable electrochemical performance. Compared to amorphous metals, nickel offers a well-established manufacturing process and relatively lower costs, making it a popular choice for commercial batteries such as nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) cells. Despite amorphous metals exhibiting superior flexibility and higher capacity potential, nickel's proven reliability and abundant availability continue to dominate electrode applications in conventional battery systems.
Structural Differences: Amorphous Metal vs Nickel
Amorphous metals for battery electrodes exhibit a non-crystalline, disordered atomic structure, which enhances their electrochemical performance by providing more active sites and improved ion diffusion pathways compared to nickel's highly ordered crystalline structure. Nickel's crystalline lattice offers structural stability but limits ion transport and surface reaction kinetics due to fewer defects and grain boundaries. The absence of long-range order in amorphous metals leads to superior corrosion resistance and mechanical flexibility, crucial for high-performance electrodes in energy storage applications.
Electrochemical Performance Comparison
Amorphous metals exhibit superior electrochemical performance compared to nickel for battery electrodes due to their disordered atomic structure, which enhances ion diffusion and increases active surface area. These materials demonstrate higher charge-discharge capacity, improved cycling stability, and reduced degradation rates under repeated electrochemical reactions. Nickel electrodes typically show lower capacity retention and slower kinetics, making amorphous metals more favorable for high-performance energy storage applications.
Energy Density and Efficiency Benefits
Amorphous metal electrodes exhibit higher energy density compared to conventional nickel electrodes due to their disordered atomic structure, which allows for increased electrochemical active sites and improved ion diffusion rates. The enhanced efficiency of amorphous metals arises from reduced crystalline grain boundaries, minimizing electron resistance and promoting faster charge-discharge cycles. These properties enable batteries with amorphous metal electrodes to achieve superior performance in terms of capacity retention and overall energy output.
Durability and Charge-Discharge Cycling
Amorphous metals exhibit superior durability and enhanced charge-discharge cycling stability compared to nickel electrodes due to their disordered atomic structure, which minimizes crack formation and mechanical degradation. Nickel electrodes tend to suffer from structural changes and capacity fading over extended cycling, reducing their lifespan in batteries. The high corrosion resistance and uniform ion diffusion properties of amorphous metals contribute to maintaining electrode integrity and electrochemical performance during repeated cycles.
Manufacturing and Scalability Considerations
Amorphous metal electrodes offer superior corrosion resistance and uniform structure, enhancing battery performance, but their manufacturing involves complex rapid cooling techniques that can hinder large-scale production. Nickel electrodes benefit from well-established, cost-effective fabrication processes and abundant material availability, making them more scalable for commercial battery manufacturing. Balancing the trade-offs between the advanced properties of amorphous metals and the production efficiency of nickel is critical for optimizing electrode scalability and cost-efficiency in battery manufacturing.
Environmental Impact and Sustainability
Amorphous metal electrodes demonstrate superior environmental benefits compared to nickel, as their production involves lower energy consumption and reduced emissions due to their unique disordered atomic structure, which requires less intensive processing. Nickel mining and processing pose significant environmental challenges, including habitat disruption, high water usage, and toxic waste generation, raising sustainability concerns. The increased recyclability and longer lifespan of amorphous metal electrodes contribute to lowering the overall ecological footprint of batteries, enhancing sustainable energy storage solutions.
Future Prospects for Battery Innovation
Amorphous metals offer superior corrosion resistance and higher electrochemical stability compared to conventional nickel electrodes, enhancing battery lifespan and performance. Their unique atomic structure allows for faster ion diffusion and improved charge-discharge cycles, making them promising candidates for next-generation energy storage. Future battery innovation is likely to leverage amorphous metal electrodes to meet increasing demands for high-capacity, durable, and efficient power sources.
Infographic: Amorphous metal vs Nickel for Battery electrode
 azmater.com
azmater.com