Lead alloys offer superior corrosion resistance and better machinability compared to chromium alloys, which provide higher hardness and wear resistance. Chromium's enhanced strength and oxidation resistance make it ideal for high-temperature applications, while lead's density and malleability suit it for radiation shielding and battery components.
Table of Comparison
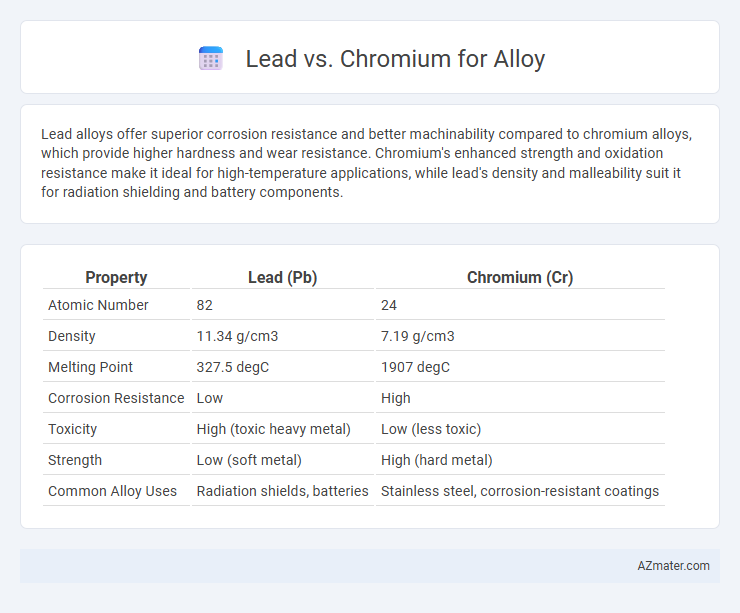
| Property | Lead (Pb) | Chromium (Cr) |
|---|---|---|
| Atomic Number | 82 | 24 |
| Density | 11.34 g/cm3 | 7.19 g/cm3 |
| Melting Point | 327.5 degC | 1907 degC |
| Corrosion Resistance | Low | High |
| Toxicity | High (toxic heavy metal) | Low (less toxic) |
| Strength | Low (soft metal) | High (hard metal) |
| Common Alloy Uses | Radiation shields, batteries | Stainless steel, corrosion-resistant coatings |
Introduction to Alloying Elements
Lead and chromium serve distinct roles in alloying due to their contrasting properties. Lead is primarily added to alloys to enhance machinability and corrosion resistance, often used in brass and solder applications. Chromium significantly improves hardness, wear resistance, and oxidation resistance, making it essential in stainless steel and other high-performance alloys.
Overview of Lead and Chromium in Metallurgy
Lead and chromium serve distinct roles in metallurgy, with lead primarily valued for its low melting point, corrosion resistance, and ability to improve machinability in alloys such as solder and batteries. Chromium is renowned for its hardness, high melting point, and exceptional corrosion resistance, particularly in stainless steel and superalloys, enhancing strength and durability. The contrasting properties of lead and chromium cater to diverse industrial applications, optimizing alloy performance based on specific mechanical and chemical requirements.
Physical and Chemical Properties Comparison
Lead and chromium alloys differ significantly in physical and chemical properties, affecting their industrial applications. Lead alloys exhibit low melting points around 327degC, high density at 11.34 g/cm3, and excellent corrosion resistance in acidic environments but lack mechanical strength and hardness. Chromium alloys possess higher melting points near 1907degC, lower density around 7.19 g/cm3, superior hardness, and exceptional resistance to oxidation and corrosion, making them ideal for high-temperature and structural uses.
Role of Lead in Alloy Composition
Lead in alloy composition primarily enhances machinability by improving chip formation and reducing tool wear, making it a vital additive in steel alloys for automotive and manufacturing industries. Its presence also increases corrosion resistance and lubrication properties, which contribute to extended component lifespan under harsh operating conditions. Chromium, contrastingly, is more focused on enhancing hardness, tensile strength, and oxidation resistance rather than machinability.
Advantages of Using Chromium in Alloys
Chromium enhances alloy strength and hardness while significantly improving corrosion resistance, making it ideal for stainless steel production. Its ability to form a stable oxide layer prevents surface degradation, ensuring long-lasting durability in harsh environments. Chromium alloys also exhibit superior heat tolerance and wear resistance compared to lead-containing alloys, expanding their industrial applications.
Impact on Mechanical Properties: Lead vs Chromium
Lead in alloys typically reduces tensile strength and hardness but enhances machinability and corrosion resistance due to its soft, malleable nature. Chromium significantly improves mechanical properties by increasing hardness, tensile strength, and wear resistance through the formation of a stable, protective oxide layer. The contrasting effects of lead and chromium directly influence the alloy's durability, performance, and suitability for high-stress applications.
Corrosion Resistance: Chromium’s Edge
Chromium significantly enhances alloy corrosion resistance by forming a stable, passive oxide layer that protects the metal surface from oxidation and chemical attack. Lead alloys lack this protective oxide layer, making them more prone to corrosion, especially in acidic or saline environments. Chromium's superior corrosion resistance ensures increased durability and longevity in harsh industrial and marine applications.
Environmental and Health Considerations
Lead alloys pose significant environmental and health risks due to lead's toxicity and persistence, leading to bioaccumulation and neurological damage in humans. Chromium alloys, particularly those using hexavalent chromium, also present health hazards such as respiratory issues and carcinogenic effects, but trivalent chromium forms are less toxic and more environmentally stable. Selecting alloys requires careful assessment of lead's higher toxicity and environmental persistence versus chromium's variable toxicity depending on its chemical state.
Industrial Applications and Performance
Lead alloys excel in corrosion resistance and machinability, making them ideal for bearing and battery grid applications in the automotive and aerospace industries. Chromium alloys provide superior hardness, wear resistance, and high-temperature stability, which enhances performance in cutting tools, turbine blades, and structural components in heavy machinery. Industrial sectors prioritize chromium for durability under extreme conditions, while lead remains favored for its cost-effectiveness and chemical stability in specialized environments.
Choosing the Right Alloying Element: Lead or Chromium?
Choosing between lead and chromium as alloying elements depends on the desired properties and application requirements. Lead enhances machinability and corrosion resistance in brass alloys but reduces strength and can pose environmental and health risks due to its toxicity. Chromium improves hardness, wear resistance, and corrosion resistance in stainless steel and other alloys, making it ideal for structural and high-performance applications where durability is critical.
Infographic: Lead vs Chromium for Alloy
 azmater.com
azmater.com