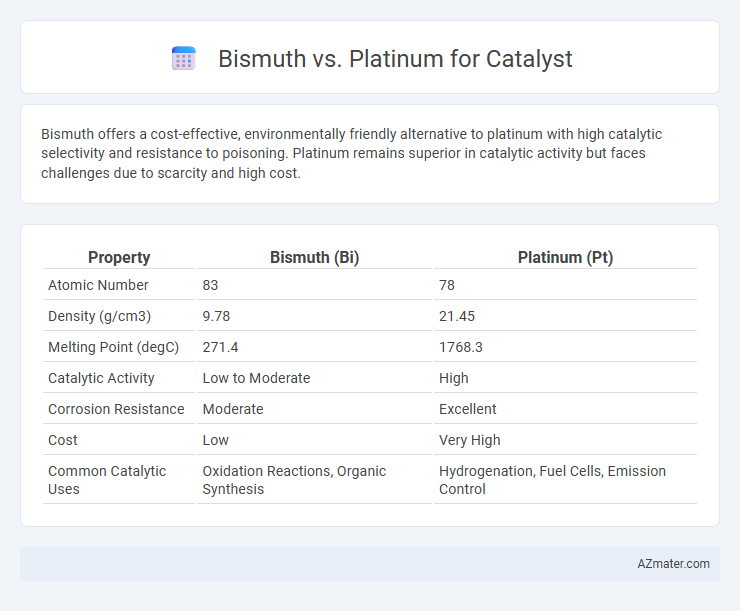
Bismuth offers a cost-effective, environmentally friendly alternative to platinum with high catalytic selectivity and resistance to poisoning. Platinum remains superior in catalytic activity but faces challenges due to scarcity and high cost.
Table of Comparison
| Property | Bismuth (Bi) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 83 | 78 |
| Density (g/cm3) | 9.78 | 21.45 |
| Melting Point (degC) | 271.4 | 1768.3 |
| Catalytic Activity | Low to Moderate | High |
| Corrosion Resistance | Moderate | Excellent |
| Cost | Low | Very High |
| Common Catalytic Uses | Oxidation Reactions, Organic Synthesis | Hydrogenation, Fuel Cells, Emission Control |
Introduction to Bismuth and Platinum Catalysts
Bismuth and platinum catalysts exhibit distinct chemical properties that influence their application in industrial processes, with platinum known for its exceptional catalytic activity and stability in reactions such as hydrogenation and oxidation. Bismuth catalysts, often used in selective organic syntheses, offer environmental benefits due to lower toxicity and cost, making them valuable in green chemistry. The choice between bismuth and platinum catalysts depends on factors like reaction specificity, operational conditions, and economic considerations, with platinum favored for high-performance catalytic converters and bismuth gaining attention in pharmaceuticals and fine chemical production.
Chemical Properties Relevant to Catalysis
Bismuth displays lower electronegativity and a larger atomic radius than platinum, influencing its electron density and surface adsorption characteristics critical for catalysis. Platinum's high electron affinity and d-orbital availability enable superior adsorption and activation of reactants, making it highly effective for hydrogenation and oxidation reactions. Bismuth's lower catalytic activity is offset by its resistance to poisoning and environmental stability, often employed in bimetallic catalysts to enhance selectivity and reduce reaction overpotentials.
Common Industrial Applications
Bismuth is primarily used as a catalyst in selective oxidation and organic synthesis processes, offering a non-toxic and cost-effective alternative in the pharmaceutical and polymer industries. Platinum, known for its exceptional catalytic activity and durability, dominates automotive catalytic converters, fuel cells, and refining processes, enabling efficient emission control and hydrogen production. While platinum excels in high-temperature and hydrogenation reactions, bismuth's niche applications leverage its environmental friendliness and unique electronic properties.
Catalytic Efficiency and Selectivity
Bismuth exhibits enhanced catalytic selectivity in reactions such as CO2 reduction due to its unique electronic structure and ability to suppress hydrogen evolution, making it highly efficient in targeted conversions. Platinum demonstrates superior catalytic efficiency in hydrogenation and oxidation reactions owing to its exceptional surface activity and durability, yet often suffers from lower selectivity due to competing side reactions. Comparing both, bismuth favors selective pathways with lower energy barriers while platinum offers higher overall reaction rates but with less precise product control.
Environmental Impact and Toxicity
Bismuth catalysts exhibit significantly lower environmental toxicity compared to platinum, as bismuth is non-toxic and biodegradable, reducing ecological risks during manufacturing and disposal. Platinum mining and refining contribute to considerable environmental degradation and pose health risks due to heavy metal exposure, raising concerns about sustainability and safety. Utilizing bismuth-based catalysts can minimize hazardous waste and lower the carbon footprint, enhancing green chemistry practices in catalytic applications.
Cost and Availability Comparison
Bismuth offers a significantly lower cost compared to platinum, making it an economically attractive catalyst choice for industrial applications. Its greater abundance in the Earth's crust enhances availability, reducing supply chain risks associated with rare platinum resources. Despite platinum's superior catalytic efficiency, bismuth's affordability and accessibility provide a compelling alternative for cost-sensitive catalytic processes.
Durability and Reusability in Catalytic Processes
Bismuth catalysts demonstrate superior durability in harsh catalytic environments due to their resistance to oxidation and poisoning compared to platinum, which often suffers from sintering and deactivation over time. The reusability of bismuth-based catalysts is enhanced by their stable electronic structure and lower cost, allowing for efficient regeneration and multiple catalytic cycles without significant loss in activity. Platinum catalysts, while highly active, face challenges in maintaining long-term stability and require more frequent replacement or complex regeneration processes, impacting overall catalyst lifecycle efficiency.
Recent Advances in Bismuth and Platinum Catalysts
Recent advances in bismuth catalysts have demonstrated enhanced selectivity and cost-effectiveness for CO2 reduction reactions, benefiting from bismuth's low toxicity and earth abundance. Platinum catalysts, renowned for their exceptional activity in hydrogen evolution and fuel cell applications, continue to see improvements through nano-structuring and alloying strategies that boost durability and catalytic efficiency. Comparative studies indicate bismuth's potential as a sustainable alternative to platinum in certain catalytic processes, especially where high selectivity and environmental considerations are prioritized.
Challenges and Limitations
Bismuth catalysts face challenges such as low electrical conductivity and limited active sites, which reduce their overall catalytic efficiency compared to platinum. Platinum, although highly effective with excellent catalytic activity and stability, suffers from high cost and scarcity, limiting its large-scale applications. Both materials experience issues related to durability under harsh reaction conditions, but research focuses on improving bismuth's conductivity and overcoming platinum's economic constraints.
Future Prospects and Research Directions
Bismuth-based catalysts exhibit promising future prospects due to their lower cost, environmental friendliness, and unique electronic properties that enhance selectivity in chemical reactions compared to platinum. Research directions focus on improving the stability and catalytic efficiency of bismuth nanostructures through doping, alloying, and surface modification techniques. Advances in computational modeling and in-situ characterization aim to better understand reaction mechanisms, facilitating the design of bismuth catalysts that could potentially rival platinum in fuel cells and electrochemical applications.
Infographic: Bismuth vs Platinum for Catalyst
 azmater.com
azmater.com