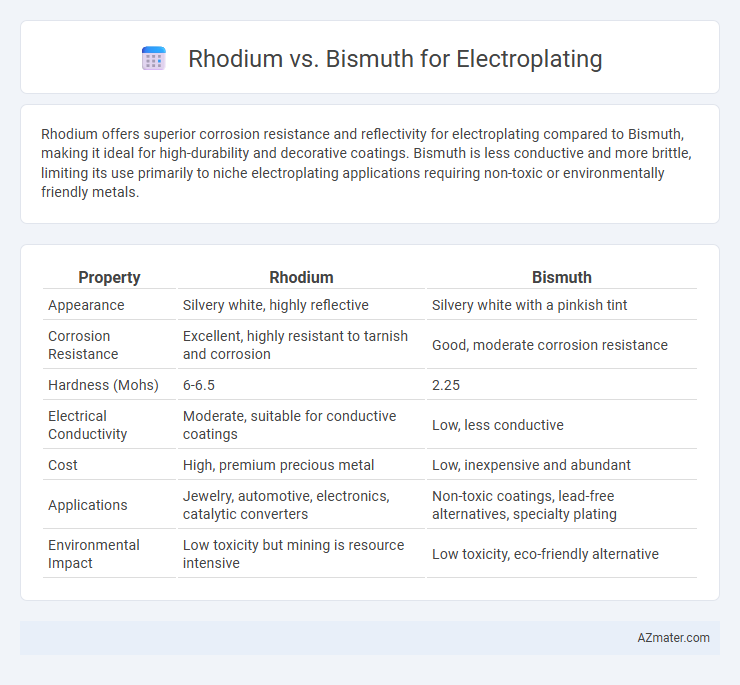
Rhodium offers superior corrosion resistance and reflectivity for electroplating compared to Bismuth, making it ideal for high-durability and decorative coatings. Bismuth is less conductive and more brittle, limiting its use primarily to niche electroplating applications requiring non-toxic or environmentally friendly metals.
Table of Comparison
| Property | Rhodium | Bismuth |
|---|---|---|
| Appearance | Silvery white, highly reflective | Silvery white with a pinkish tint |
| Corrosion Resistance | Excellent, highly resistant to tarnish and corrosion | Good, moderate corrosion resistance |
| Hardness (Mohs) | 6-6.5 | 2.25 |
| Electrical Conductivity | Moderate, suitable for conductive coatings | Low, less conductive |
| Cost | High, premium precious metal | Low, inexpensive and abundant |
| Applications | Jewelry, automotive, electronics, catalytic converters | Non-toxic coatings, lead-free alternatives, specialty plating |
| Environmental Impact | Low toxicity but mining is resource intensive | Low toxicity, eco-friendly alternative |
Introduction to Rhodium and Bismuth in Electroplating
Rhodium and bismuth are both utilized in electroplating to enhance surface properties, with rhodium renowned for its exceptional hardness, corrosion resistance, and brilliant, reflective finish, making it ideal for jewelry and automotive parts. Bismuth, less common in electroplating, serves primarily as an environmentally friendly substitute in lead-free alloys and coatings, valued for its non-toxicity and moderate corrosion resistance. The choice between rhodium and bismuth depends on the application requirements, balancing durability, environmental impact, and aesthetic appeal in electroplated products.
Chemical Properties Overview
Rhodium exhibits exceptional corrosion resistance, high reflectivity, and excellent hardness due to its noble metal status and stable oxidation state of +3, making it ideal for electroplating applications requiring durability and conductivity. In contrast, bismuth, a post-transition metal with a low toxicity profile and relatively low melting point, offers good electrical conductivity but lower hardness and corrosion resistance compared to rhodium. The chemical inertness and ability of rhodium to form stable complexes make it preferable for high-performance electroplating, whereas bismuth's unique electronic structure lends itself to specialized coatings needing non-toxicity and moderate protective qualities.
Electroplating Process Differences
Rhodium electroplating involves using a strong acidic bath with rhodium salts, typically requiring higher currents and precise temperature control to achieve thin, durable, and highly reflective coatings. Bismuth electroplating uses a less aggressive, often alkaline solution, allowing for thicker deposits with good corrosion resistance but lower reflectivity compared to rhodium. The process for rhodium demands careful monitoring of pH and plating time to avoid brittleness, while bismuth plating prioritizes uniformity and ductility in the final layer.
Surface Finish and Aesthetic Comparison
Rhodium electroplating offers a highly reflective, bright white surface finish with excellent corrosion resistance, making it ideal for jewelry and decorative applications requiring a mirror-like shine. Bismuth coatings provide a unique iridescent or matte finish with excellent environmental compatibility but lack the high luster and durability of rhodium. For applications prioritizing superior aesthetic brilliance and wear resistance, rhodium remains the preferred choice, while bismuth suits niche uses valuing non-toxic and distinctive surface appearances.
Electrical Conductivity Performance
Rhodium offers superior electrical conductivity in electroplating applications, making it ideal for high-performance electronic connectors and contacts. Bismuth, while less conductive than rhodium, is valued for its non-toxic and environmentally friendly properties but is typically used where electrical performance is not the primary concern. The choice between rhodium and bismuth largely depends on the need for low electrical resistance versus sustainability considerations in plating processes.
Corrosion Resistance and Durability
Rhodium offers superior corrosion resistance and exceptional durability in electroplating applications, making it ideal for jewelry and automotive components exposed to harsh environments. Bismuth, while less durable and more prone to corrosion, is often chosen for its eco-friendly and non-toxic properties, but it requires protective coatings to enhance its longevity. Electroplated rhodium layers provide a harder, more wear-resistant surface compared to bismuth, ensuring prolonged lifespan and sustained aesthetic appeal.
Cost and Availability Analysis
Rhodium, a rare and highly valuable platinum-group metal, commands a significantly higher cost due to its scarcity and limited annual production of approximately 30 metric tons, making it one of the most expensive materials for electroplating. Bismuth, by contrast, is more abundant with global production exceeding 20,000 metric tons annually and is significantly cheaper, making it a cost-effective alternative for decorative and corrosion-resistant plating. The high price and limited availability of rhodium often restrict its use to high-end applications, while bismuth's accessibility supports its wider adoption in industrial electroplating processes.
Environmental and Health Considerations
Rhodium electroplating offers superior corrosion resistance and brightness but poses significant environmental and health risks due to its toxic plating baths containing hazardous chemicals like hexavalent chromium. Bismuth, a non-toxic, environmentally friendly alternative, provides safer waste disposal and reduced exposure to harmful substances, though it may lack some durability compared to rhodium coatings. Choosing bismuth plating minimizes ecological impact and operator health hazards, aligning with sustainable manufacturing practices.
Industrial Applications and Use Cases
Rhodium is widely used in electroplating for automotive catalytic converters and jewelry due to its exceptional corrosion resistance, high reflectivity, and hardness, making it ideal for decorative and protective coatings in harsh industrial environments. Bismuth electroplating finds niche applications in electronic components and medical devices because of its non-toxic, environmentally friendly properties and excellent solderability. Industrial use cases favor rhodium for durability and wear resistance, while bismuth is preferred where eco-friendly and lead-free alternatives are critical.
Choosing the Right Metal for Electroplating Needs
Rhodium offers superior corrosion resistance, exceptional hardness, and a brilliant reflective finish, making it ideal for high-end jewelry and automotive trim electroplating applications. Bismuth provides a cost-effective and environmentally friendly alternative with moderate corrosion resistance and good electrical conductivity, suitable for decorative and industrial coatings where sustainability is prioritized. Selecting rhodium or bismuth hinges on balancing factors like durability, appearance, budget, and ecological impact to meet specific electroplating requirements.
Infographic: Rhodium vs Bismuth for Electroplating
 azmater.com
azmater.com