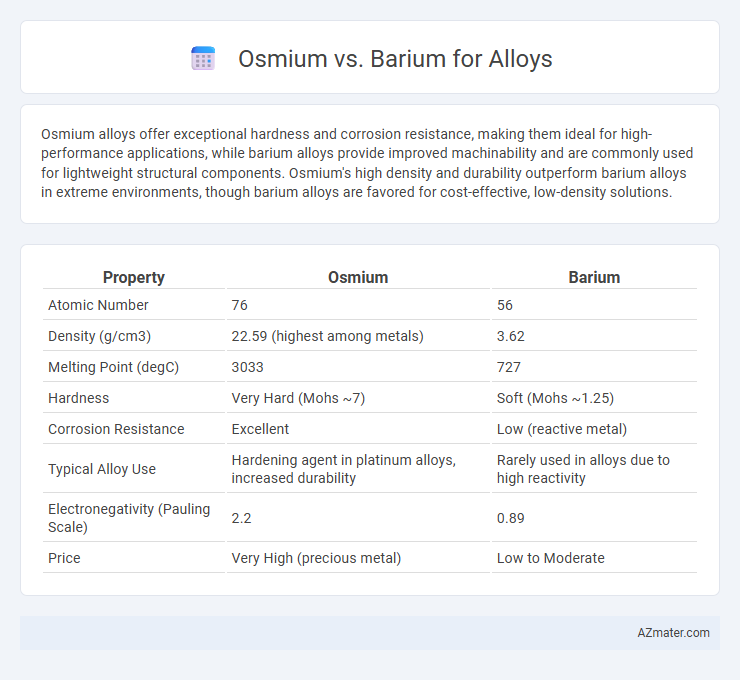
Osmium alloys offer exceptional hardness and corrosion resistance, making them ideal for high-performance applications, while barium alloys provide improved machinability and are commonly used for lightweight structural components. Osmium's high density and durability outperform barium alloys in extreme environments, though barium alloys are favored for cost-effective, low-density solutions.
Table of Comparison
| Property | Osmium | Barium |
|---|---|---|
| Atomic Number | 76 | 56 |
| Density (g/cm3) | 22.59 (highest among metals) | 3.62 |
| Melting Point (degC) | 3033 | 727 |
| Hardness | Very Hard (Mohs ~7) | Soft (Mohs ~1.25) |
| Corrosion Resistance | Excellent | Low (reactive metal) |
| Typical Alloy Use | Hardening agent in platinum alloys, increased durability | Rarely used in alloys due to high reactivity |
| Electronegativity (Pauling Scale) | 2.2 | 0.89 |
| Price | Very High (precious metal) | Low to Moderate |
Introduction to Osmium and Barium in Alloying
Osmium, a dense and corrosion-resistant transition metal, is valued in alloying for its hardness and durability, often enhancing wear resistance and stability in extreme conditions. Barium, a softer alkaline earth metal, is rarely alloyed directly but serves important roles in improving the properties of alloys through barium compounds, such as refining microstructures or acting as deoxidizers. The contrasting characteristics of osmium and barium reflect their distinct applications in metallurgy, with osmium contributing to strength and longevity, while barium primarily influences alloy processing and performance indirectly.
Chemical Properties of Osmium vs Barium
Osmium exhibits exceptional chemical stability and resistance to corrosion due to its dense atomic structure and noble metal characteristics, making it ideal for high-durability alloys. In contrast, barium is highly reactive, especially with oxygen and water, which limits its use in alloys to situations requiring chemical reactivity or electrical applications. The distinct oxidation states of osmium (commonly +4 and +8) contribute to its robust alloy performance compared to barium's more reactive nature and predominant +2 oxidation state.
Physical Characteristics and Density Comparison
Osmium exhibits extreme density at 22.59 g/cm3, making it one of the densest naturally occurring elements, while barium has a significantly lower density of 3.62 g/cm3, resulting in heavier yet more brittle osmium alloys versus lighter, more malleable barium-based alloys. Osmium's high melting point of 3033degC contrasts with barium's much lower melting point at 727degC, impacting alloy stability at elevated temperatures. The hardness and brittleness of osmium alloys lend themselves to specialized industrial applications, while barium alloys offer flexibility and lower weight for different engineering uses.
Reactivity and Stability in Alloy Mixtures
Osmium exhibits exceptional chemical stability and low reactivity, making it highly suitable for alloys requiring corrosion resistance and durability under extreme conditions. In contrast, barium is highly reactive, especially with oxygen and moisture, which limits its use in stable alloy mixtures and necessitates protective environments or coatings. The superior stability of osmium alloys ensures longevity and consistent performance in industrial applications where alloy integrity is critical.
Melting Points and Thermal Behavior
Osmium exhibits a melting point of approximately 3033degC, significantly higher than barium's melting point of about 727degC, making osmium alloys suitable for high-temperature applications. Osmium's exceptional thermal stability and low thermal expansion contribute to superior performance in extreme environments, whereas barium alloys display greater thermal expansion and lower heat resistance. These contrasting thermal behaviors influence alloy selection for industries demanding heat resistance and structural integrity under intense temperature conditions.
Alloying Techniques: Osmium vs Barium
Osmium alloys are primarily produced through powder metallurgy and high-temperature sintering techniques that preserve its exceptional hardness and density, making it ideal for specialized applications like electrical contacts and fountain pen tips. In contrast, barium is rarely alloyed directly due to its high reactivity and softness; instead, it is often used as an additive or doping agent in metal alloys, typically incorporated through melting and casting processes under inert atmospheres to prevent oxidation. The difference in alloying techniques between osmium and barium significantly affects their structural properties, with osmium alloys emphasizing durability and density while barium-modified alloys focus on enhancing magnetic and electronic characteristics.
Mechanical Strength and Durability Factors
Osmium alloys exhibit exceptional mechanical strength and durability due to osmium's high density and remarkable hardness, making them ideal for applications requiring extreme wear resistance and structural integrity. Barium alloys, although less dense and strong, offer improved corrosion resistance and flexibility, suited for less demanding mechanical environments. The choice between osmium and barium alloys depends on the specific balance needed between ultimate tensile strength, hardness, and corrosion resistance in industrial applications.
Industrial Applications and Use Cases
Osmium alloys are prized in industrial applications requiring extreme hardness, high density, and wear resistance, notably in electrical contacts, fountain pen nibs, and specialized surgical instruments due to osmium's superior corrosion resistance and melting point. Barium, often used in alloy form with metals like nickel or aluminum, finds extensive use in electronics and glass manufacturing, especially as a component in barium ferrite magnets and as a deoxidizer or scavenger in metallurgical processes. The distinct physical and chemical properties of osmium and barium alloys drive their specialized roles, with osmium favored for durability under harsh conditions and barium for magnetic and chemical reactivity applications.
Environmental and Safety Considerations
Osmium alloys are valued for their extreme hardness and density but pose significant environmental and safety risks due to osmium tetroxide, a highly toxic and volatile compound formed upon exposure to air. Barium alloys, while less dense and hard, often contain barium which is toxic and requires careful handling to prevent soil and water contamination, especially in industrial applications. Choosing between osmium and barium alloys necessitates strict adherence to safety protocols and environmental regulations to mitigate the hazardous effects associated with each metal.
Cost Analysis and Market Availability
Osmium, a rare and dense platinum-group metal, commands a significantly higher price than barium, primarily due to its scarcity and complex extraction process, affecting its feasibility in bulk alloy production. Barium, more abundant and less costly, is widely available and commonly used in alloys for electronic and aerospace applications, offering better market accessibility and cost-efficiency. Cost analysis reveals osmium alloys are niche and expensive, while barium-based alloys support broader industrial use with scalable supply chains.
Infographic: Osmium vs Barium for Alloys
 azmater.com
azmater.com