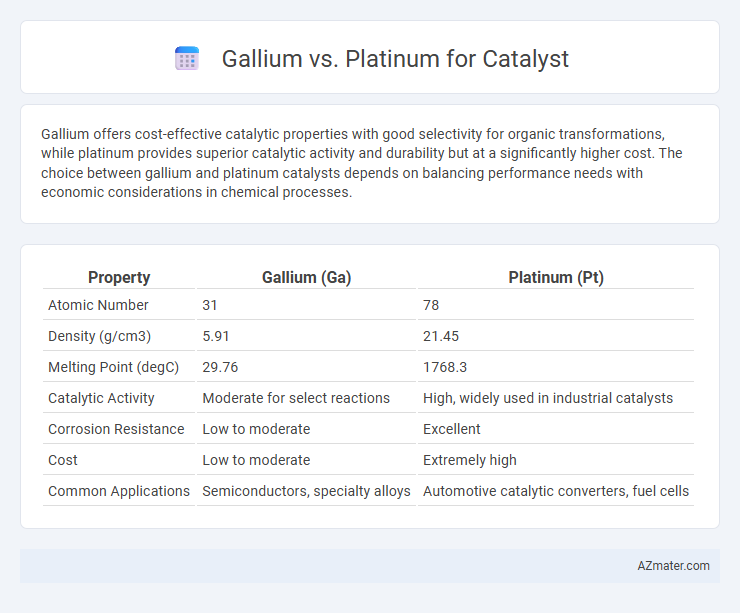
Gallium offers cost-effective catalytic properties with good selectivity for organic transformations, while platinum provides superior catalytic activity and durability but at a significantly higher cost. The choice between gallium and platinum catalysts depends on balancing performance needs with economic considerations in chemical processes.
Table of Comparison
| Property | Gallium (Ga) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 31 | 78 |
| Density (g/cm3) | 5.91 | 21.45 |
| Melting Point (degC) | 29.76 | 1768.3 |
| Catalytic Activity | Moderate for select reactions | High, widely used in industrial catalysts |
| Corrosion Resistance | Low to moderate | Excellent |
| Cost | Low to moderate | Extremely high |
| Common Applications | Semiconductors, specialty alloys | Automotive catalytic converters, fuel cells |
Introduction to Catalysts: Gallium vs Platinum
Gallium and platinum serve distinct roles as catalysts, with platinum being a well-established noble metal catalyst known for its exceptional activity and stability in hydrogenation and oxidation reactions. Gallium, a post-transition metal, is gaining attention for its potential in catalysis due to its ability to form unique liquid metal alloys and activate small molecules under mild conditions. The choice between gallium and platinum depends on factors such as catalytic efficiency, cost, and specific reaction environments.
Chemical Properties Relevant to Catalysis
Gallium exhibits unique Lewis acidity and a moderate melting point, enabling its use as a catalyst in selective hydrogenation and coupling reactions, while maintaining lower toxicity compared to many heavy metals. Platinum offers exceptional catalytic activity and durability due to its electronic structure, facilitating efficient surface adsorption and activation of reactants in processes like hydrogenation and oxidation. The distinct electronic configurations and oxidation states of gallium and platinum significantly influence their catalytic selectivity and resistance to poisoning in industrial applications.
Abundance and Availability: Gallium and Platinum Sources
Gallium is primarily extracted as a byproduct of aluminum and zinc mining, with major sources found in bauxite and sphalerite ores, making it more abundant and widely available compared to platinum. Platinum, a rare noble metal, is mainly sourced from underground mines in South Africa, Russia, and Canada, resulting in limited reserves and higher extraction costs. The greater abundance and more accessible production of gallium contribute to its potential as a cost-effective alternative catalyst material in various industrial applications.
Catalytic Efficiency and Reaction Rates
Gallium catalysts often exhibit higher selectivity and enhanced catalytic efficiency in specific reactions like hydrogenation compared to platinum, particularly due to their ability to activate substrates at lower temperatures. Platinum, renowned for its excellent reaction rates, maintains superior performance in a broad range of catalytic processes, including oxidation and reforming reactions, due to its robust surface properties and electronic structure. The choice between gallium and platinum as catalysts depends on the desired reaction pathway and operating conditions, with gallium offering cost-effective alternatives in selective catalysis and platinum excelling in high-activity scenarios.
Selectivity in Chemical Reactions
Gallium catalysts exhibit higher selectivity in hydrogenation reactions compared to platinum, particularly in producing olefins and aromatics due to their unique electronic properties and reduced over-hydrogenation tendencies. Platinum catalysts, while highly active, often lead to lower selectivity because of their strong adsorption and hydrogen spillover effects, which promote undesired side reactions. The choice between gallium and platinum significantly impacts product yield and purity in processes such as alkene hydrogenation and dehydrogenation, where selective catalysis is critical.
Thermal Stability and Operating Conditions
Gallium-based catalysts exhibit superior thermal stability compared to platinum, enabling them to maintain catalytic activity at higher temperatures without significant deactivation or sintering. Platinum catalysts typically operate effectively at moderate temperatures but suffer from reduced thermal tolerance, leading to faster degradation under harsh conditions. Gallium's enhanced resistance to thermal stress makes it ideal for high-temperature industrial processes where catalyst longevity and consistent performance are critical.
Environmental Impact and Sustainability
Gallium-based catalysts offer a lower environmental impact compared to platinum due to their abundance and lower energy-intensive extraction processes, resulting in decreased ecological footprint. Platinum mining and refining involve significant greenhouse gas emissions and habitat disruption, challenging sustainability efforts in catalytic applications. Gallium's recyclability and potential for reduced resource depletion make it a promising sustainable alternative for catalyst development.
Economic Considerations: Cost and Scalability
Gallium offers a cost-effective alternative to platinum as a catalyst due to its lower market price and greater abundance, which significantly reduces raw material expenses in large-scale applications. The scalability of gallium-based catalysts benefits from simpler extraction and processing techniques compared to platinum, enabling more flexible and economically viable production methods. Economic analyses emphasize that adopting gallium catalysts can enhance cost efficiency in industrial processes, particularly in sectors requiring extensive catalytic surfaces or high-volume catalyst usage.
Applications in Industry: Case Studies
Gallium-based catalysts demonstrate enhanced selectivity and lower cost in hydrogenation reactions within the pharmaceutical industry, significantly improving drug synthesis efficiency. Platinum catalysts remain dominant in automotive catalytic converters due to their superior durability and tolerance to high temperatures, crucial for reducing vehicular emissions. Case studies reveal gallium's growing role in fine chemical production, while platinum's robustness underpins large-scale industrial processes in petrochemical refining and fuel cell technology.
Future Prospects and Research Directions
Gallium shows promising future prospects as a more abundant and cost-effective alternative to platinum in catalyst applications, particularly in hydrogen production and CO2 reduction. Research directions focus on enhancing gallium-based catalyst stability and catalytic efficiency through alloying with transition metals and optimizing nanostructures. Innovations in gallium catalysts may lead to sustainable, scalable solutions for renewable energy conversion, potentially rivaling platinum's catalytic performance.
Infographic: Gallium vs Platinum for Catalyst
 azmater.com
azmater.com